INOTUZUMAB OZOGAMICIN IN COMBINATION WITH LOW-INTENSITY CHEMOTHERAPY (MINI-HYPER-CVD) FOR THE FRONTLINE THERAPY IN ELDERLY PATIENTS (?60 YEARS) WITH ACUTE LYMPHOBLASTIC LEUKEMIA (ALL)
(Abstract release date: 05/21/15)
EHA Library. Jabbour E. 06/12/15; 103176; S114
Disclosure(s): MD Anderson Cancer CenterLeukemia

Dr. Elias Jabbour
Contributions
Contributions
Abstract
Abstract: S114
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:15 to 12.06.2015 12:30
Location: Room C1
Background
Older patients (pts) with ALL have a significantly worse outcome. This is primarily due to poor tolerance of intensive chemotherapy. Addition of targeted non-myelosuppressive therapy to effective low-intensity chemotherapy might improve outcome. CD22 expression occurs in >90% of pts with ALL. Inotuzumab ozogamicin (INO) is a CD22 monoclonal antibody bound to a toxin, calecheamicin, and has shown single-agent activity in relapsed/refractory ALL (Kantarjian et al. Lancet Oncology 2012).
Aims
To determine the efficacy of INO in combination with mini-hper-CVD assessed by objective response rate, progression-free, and overall survival and to assess the side effects of this treatment.
Methods
Pts ≥60 years (yrs) with newly-diagnosed B-cell ALL were eligible. The chemotherapy was lower intensity than conventional hyper-CVAD and referred to as mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses). Rituximab and intrathecal chemotherapy were given for first 4 courses. INO was given on Day 3 of each of the first 4 courses. The first 6 pts received 1.3 mg/m2 for cycle 1 followed by 0.8 mg/m2 for subsequent cycles; Pts 7 onwards received 1.8 mg/m2 for Cycle 1 followed by 1.3 mg/m2 for subsequent cycles.
Results
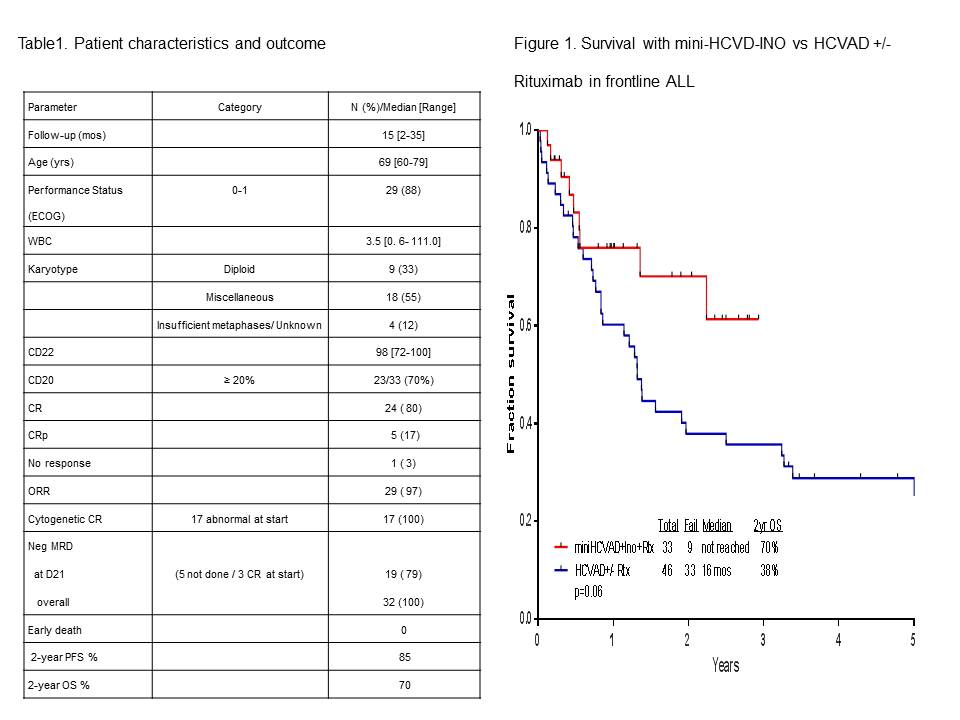
Thirty-three pts (20 men, 13 women) have been treated so far. Pts characteristics and outcome are summarized in Table 1. Median age is 69 yrs (range, 60-79). Median follow-up is 15 months (mos) (range, 2-35). Of the 30 pts evaluable for response (three pts started in CR; two achieved with single-agent steroids and one with one course of HCVAD), 29 pts (97%) achieved CR/CRp (24 CR, 5 CRp). All pts achieving CR have also achieved flow-cytometric MRD negative status, in 79% at the time of CR achievement. Grade 3-4 toxicities included infections (n= 29; 88%), prolonged thrombocytopenia (n= 25; 76%), hyperglycemia (n= 17; 52%); hypokalemia (n=11; 33%); increased bilirubin (n= 8; 24%); increased ALT (n= 7; 21%), and intracranial hemorrhage (n=4; 12%). Grade 2 veno-occlusive occurred in 2 (7%) pts (7%). At the last follow-up, 24 (73%) pts are alive, and 23 (70%) are in CR. Nine (27%) pts died: 1 had primary refractory ALL and died after the first salvage; 2 relapsed after receiving 3 and 2 courses only due to prolonged myelossuppression and died of disease progression; and 6 died in CR from pneumonia complications (n=1), sepsis and multiple organ failure (n=1), gun-shot wound (n=1), renal failure and metabolic encephalopathy (n=1), complications due to dementia (n=1), and unknown (n=1). One pt received allogeneic stem cell transplantation. The 2-year progression-free survival and overall survival rates were 85% and 70%, respectively. The mini-hyper-CVD (n= 33) appears superior to the historical HCVAD +/- rituximab (n=46) in similar patient population (2-year survival rates 78% and 38%, respectively; Figure 1).
Summary
The combination of INO with low-intensity mini-hyper-CVD chemotherapy is safe and shows encouraging results (96% CR/CRp) in the frontline setting in older pts with ALL. These results appear to be better than those achieved with a chemotherapy alone approach and may become the new standard of care for frontline treatment of older pts with ALL.
Keyword(s): ALL
Session topic: ALL clinical trials
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:15 to 12.06.2015 12:30
Location: Room C1
Background
Older patients (pts) with ALL have a significantly worse outcome. This is primarily due to poor tolerance of intensive chemotherapy. Addition of targeted non-myelosuppressive therapy to effective low-intensity chemotherapy might improve outcome. CD22 expression occurs in >90% of pts with ALL. Inotuzumab ozogamicin (INO) is a CD22 monoclonal antibody bound to a toxin, calecheamicin, and has shown single-agent activity in relapsed/refractory ALL (Kantarjian et al. Lancet Oncology 2012).
Aims
To determine the efficacy of INO in combination with mini-hper-CVD assessed by objective response rate, progression-free, and overall survival and to assess the side effects of this treatment.
Methods
Pts ≥60 years (yrs) with newly-diagnosed B-cell ALL were eligible. The chemotherapy was lower intensity than conventional hyper-CVAD and referred to as mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses). Rituximab and intrathecal chemotherapy were given for first 4 courses. INO was given on Day 3 of each of the first 4 courses. The first 6 pts received 1.3 mg/m2 for cycle 1 followed by 0.8 mg/m2 for subsequent cycles; Pts 7 onwards received 1.8 mg/m2 for Cycle 1 followed by 1.3 mg/m2 for subsequent cycles.
Results
Thirty-three pts (20 men, 13 women) have been treated so far. Pts characteristics and outcome are summarized in Table 1. Median age is 69 yrs (range, 60-79). Median follow-up is 15 months (mos) (range, 2-35). Of the 30 pts evaluable for response (three pts started in CR; two achieved with single-agent steroids and one with one course of HCVAD), 29 pts (97%) achieved CR/CRp (24 CR, 5 CRp). All pts achieving CR have also achieved flow-cytometric MRD negative status, in 79% at the time of CR achievement. Grade 3-4 toxicities included infections (n= 29; 88%), prolonged thrombocytopenia (n= 25; 76%), hyperglycemia (n= 17; 52%); hypokalemia (n=11; 33%); increased bilirubin (n= 8; 24%); increased ALT (n= 7; 21%), and intracranial hemorrhage (n=4; 12%). Grade 2 veno-occlusive occurred in 2 (7%) pts (7%). At the last follow-up, 24 (73%) pts are alive, and 23 (70%) are in CR. Nine (27%) pts died: 1 had primary refractory ALL and died after the first salvage; 2 relapsed after receiving 3 and 2 courses only due to prolonged myelossuppression and died of disease progression; and 6 died in CR from pneumonia complications (n=1), sepsis and multiple organ failure (n=1), gun-shot wound (n=1), renal failure and metabolic encephalopathy (n=1), complications due to dementia (n=1), and unknown (n=1). One pt received allogeneic stem cell transplantation. The 2-year progression-free survival and overall survival rates were 85% and 70%, respectively. The mini-hyper-CVD (n= 33) appears superior to the historical HCVAD +/- rituximab (n=46) in similar patient population (2-year survival rates 78% and 38%, respectively; Figure 1).
Summary
The combination of INO with low-intensity mini-hyper-CVD chemotherapy is safe and shows encouraging results (96% CR/CRp) in the frontline setting in older pts with ALL. These results appear to be better than those achieved with a chemotherapy alone approach and may become the new standard of care for frontline treatment of older pts with ALL.
Keyword(s): ALL

Session topic: ALL clinical trials
Abstract: S114
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:15 to 12.06.2015 12:30
Location: Room C1
Background
Older patients (pts) with ALL have a significantly worse outcome. This is primarily due to poor tolerance of intensive chemotherapy. Addition of targeted non-myelosuppressive therapy to effective low-intensity chemotherapy might improve outcome. CD22 expression occurs in >90% of pts with ALL. Inotuzumab ozogamicin (INO) is a CD22 monoclonal antibody bound to a toxin, calecheamicin, and has shown single-agent activity in relapsed/refractory ALL (Kantarjian et al. Lancet Oncology 2012).
Aims
To determine the efficacy of INO in combination with mini-hper-CVD assessed by objective response rate, progression-free, and overall survival and to assess the side effects of this treatment.
Methods
Pts ≥60 years (yrs) with newly-diagnosed B-cell ALL were eligible. The chemotherapy was lower intensity than conventional hyper-CVAD and referred to as mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses). Rituximab and intrathecal chemotherapy were given for first 4 courses. INO was given on Day 3 of each of the first 4 courses. The first 6 pts received 1.3 mg/m2 for cycle 1 followed by 0.8 mg/m2 for subsequent cycles; Pts 7 onwards received 1.8 mg/m2 for Cycle 1 followed by 1.3 mg/m2 for subsequent cycles.
Results
Thirty-three pts (20 men, 13 women) have been treated so far. Pts characteristics and outcome are summarized in Table 1. Median age is 69 yrs (range, 60-79). Median follow-up is 15 months (mos) (range, 2-35). Of the 30 pts evaluable for response (three pts started in CR; two achieved with single-agent steroids and one with one course of HCVAD), 29 pts (97%) achieved CR/CRp (24 CR, 5 CRp). All pts achieving CR have also achieved flow-cytometric MRD negative status, in 79% at the time of CR achievement. Grade 3-4 toxicities included infections (n= 29; 88%), prolonged thrombocytopenia (n= 25; 76%), hyperglycemia (n= 17; 52%); hypokalemia (n=11; 33%); increased bilirubin (n= 8; 24%); increased ALT (n= 7; 21%), and intracranial hemorrhage (n=4; 12%). Grade 2 veno-occlusive occurred in 2 (7%) pts (7%). At the last follow-up, 24 (73%) pts are alive, and 23 (70%) are in CR. Nine (27%) pts died: 1 had primary refractory ALL and died after the first salvage; 2 relapsed after receiving 3 and 2 courses only due to prolonged myelossuppression and died of disease progression; and 6 died in CR from pneumonia complications (n=1), sepsis and multiple organ failure (n=1), gun-shot wound (n=1), renal failure and metabolic encephalopathy (n=1), complications due to dementia (n=1), and unknown (n=1). One pt received allogeneic stem cell transplantation. The 2-year progression-free survival and overall survival rates were 85% and 70%, respectively. The mini-hyper-CVD (n= 33) appears superior to the historical HCVAD +/- rituximab (n=46) in similar patient population (2-year survival rates 78% and 38%, respectively; Figure 1).
Summary
The combination of INO with low-intensity mini-hyper-CVD chemotherapy is safe and shows encouraging results (96% CR/CRp) in the frontline setting in older pts with ALL. These results appear to be better than those achieved with a chemotherapy alone approach and may become the new standard of care for frontline treatment of older pts with ALL.
Keyword(s): ALL

Session topic: ALL clinical trials
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:15 to 12.06.2015 12:30
Location: Room C1
Background
Older patients (pts) with ALL have a significantly worse outcome. This is primarily due to poor tolerance of intensive chemotherapy. Addition of targeted non-myelosuppressive therapy to effective low-intensity chemotherapy might improve outcome. CD22 expression occurs in >90% of pts with ALL. Inotuzumab ozogamicin (INO) is a CD22 monoclonal antibody bound to a toxin, calecheamicin, and has shown single-agent activity in relapsed/refractory ALL (Kantarjian et al. Lancet Oncology 2012).
Aims
To determine the efficacy of INO in combination with mini-hper-CVD assessed by objective response rate, progression-free, and overall survival and to assess the side effects of this treatment.
Methods
Pts ≥60 years (yrs) with newly-diagnosed B-cell ALL were eligible. The chemotherapy was lower intensity than conventional hyper-CVAD and referred to as mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses). Rituximab and intrathecal chemotherapy were given for first 4 courses. INO was given on Day 3 of each of the first 4 courses. The first 6 pts received 1.3 mg/m2 for cycle 1 followed by 0.8 mg/m2 for subsequent cycles; Pts 7 onwards received 1.8 mg/m2 for Cycle 1 followed by 1.3 mg/m2 for subsequent cycles.
Results
Thirty-three pts (20 men, 13 women) have been treated so far. Pts characteristics and outcome are summarized in Table 1. Median age is 69 yrs (range, 60-79). Median follow-up is 15 months (mos) (range, 2-35). Of the 30 pts evaluable for response (three pts started in CR; two achieved with single-agent steroids and one with one course of HCVAD), 29 pts (97%) achieved CR/CRp (24 CR, 5 CRp). All pts achieving CR have also achieved flow-cytometric MRD negative status, in 79% at the time of CR achievement. Grade 3-4 toxicities included infections (n= 29; 88%), prolonged thrombocytopenia (n= 25; 76%), hyperglycemia (n= 17; 52%); hypokalemia (n=11; 33%); increased bilirubin (n= 8; 24%); increased ALT (n= 7; 21%), and intracranial hemorrhage (n=4; 12%). Grade 2 veno-occlusive occurred in 2 (7%) pts (7%). At the last follow-up, 24 (73%) pts are alive, and 23 (70%) are in CR. Nine (27%) pts died: 1 had primary refractory ALL and died after the first salvage; 2 relapsed after receiving 3 and 2 courses only due to prolonged myelossuppression and died of disease progression; and 6 died in CR from pneumonia complications (n=1), sepsis and multiple organ failure (n=1), gun-shot wound (n=1), renal failure and metabolic encephalopathy (n=1), complications due to dementia (n=1), and unknown (n=1). One pt received allogeneic stem cell transplantation. The 2-year progression-free survival and overall survival rates were 85% and 70%, respectively. The mini-hyper-CVD (n= 33) appears superior to the historical HCVAD +/- rituximab (n=46) in similar patient population (2-year survival rates 78% and 38%, respectively; Figure 1).
Summary
The combination of INO with low-intensity mini-hyper-CVD chemotherapy is safe and shows encouraging results (96% CR/CRp) in the frontline setting in older pts with ALL. These results appear to be better than those achieved with a chemotherapy alone approach and may become the new standard of care for frontline treatment of older pts with ALL.
Keyword(s): ALL

Session topic: ALL clinical trials
{{ help_message }}
{{filter}}


