ELTROMBOPAG ADDED TO STANDARD IMMUNOSUPPRESSION AS FIRST TREATMENT IN SEVERE APLASTIC ANEMIA
(Abstract release date: 05/21/15)
EHA Library. Townsley D. 06/14/15; 103174; S826
Disclosure(s): National Institutes of Health, National Heart, Lung, and Blood Institute
Danielle Townsley
Contributions
Contributions
Abstract
Abstract: S826
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:15 to 14.06.2015 08:30
Location: Room Stolz 2
Background
Immunosuppressive treatment (IST) for severe aplastic anemia (SAA) has a 60-65% rate of overall hematologic response (OR), of which only about 10% are complete (CR). Eltrombopag, a synthetic thrombopoietin receptor agonist (TPO-RA), has activity in refractory SAA, producing multilineage hematologic responses in about 40% of patients.
Aims
We conducted an investigator-initiated phase II, single-center trial to test the efficacy of adding eltrombopag to h-ATG/CsA (clinicaltrials.gov, NCT01623167).
Methods
Patients with treatment-naïve SAA were enrolled from July 2012 to November 2014. All subjects received standard h-ATG and CsA. To determine the optimum regimen, eltrombopag was administered at 150 mg daily to two consecutively enrolling cohorts (see Table). The primary endpoint was CR at 6 months. Both cohorts were powered to detect a ≥ 30% CR rate at 6 months for comparison with our historical rate of 10%. Our historical data suggest that CR is a surrogate for late events since it is associated with a low rate of evolution to monosomy 7/acute leukemia and correlates with excellent long-term survival.
Results
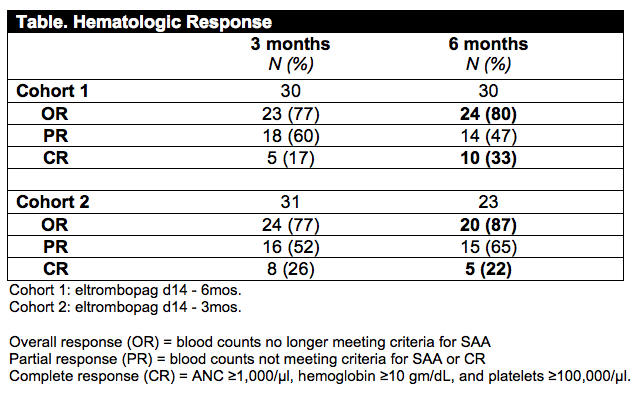
Thirty subjects enrolled in the first cohort had a median age of 39 years (12-72); thirteen (43%) had very severe disease (ANC<200/µl), and 8 (27%) had PNH clones ≥1%. Eltrombopag was well tolerated when combined with CsA, and only three patients discontinued treatment before 6 months. CR at 6 months was 33% (95% CI, 15-51%), significantly higher than our historical rate of 10% (p<0.001). OR at 6 months was 80% (CI, 65-95%), exceeding the historical rate of 60% (p=0.040). Median increases in blood counts among responders at 6 months (n=24) were: absolute neutrophil count (ANC) +1240/µl; hemoglobin +3.8 gm/dL; and platelets +87,000/µl. Median time to achieve transfusion independence was 32 days for platelets and 39 days for red cells. Serial BM biopsies showed improved cellularity in 25 cases without increased fibrosis. Median increase in BM CD34+ cells, as measured by flow cytometry, was 34-fold from baseline to 3 months, and 23-fold from baseline to 6 months (p<0.0001). Primitive hematopoietic progenitors were serially measured in 4 patients, detecting HSC (CD34+CD38-CD45RA-CD90+CD49f+Rholo) and MPP (CD34+CD38-CD45RA-CD90-CD49f-) by flow cytometry. Undetectable at baseline, these cells constituted 4-48 HSC and 1-27 MPP per 100,000 CD34+ cells 6 months after therapy. The first cohort has been followed for median 24 months (range 15-31m); there have been 3 clonal evolution events, all detected in responders: one patient had complex cytogenetics (t(3;3)(q21;q26)) with BM dysplasia followed by increased blasts, and deletion 13q in one subject and trisomy 15 in another, neither with BM dysplasia. Enrollment to the second cohort, in which eltrombopag is stopped at 3 months, is complete and 23 subjects are currently evaluable at 6 months. OR at 6 months is 87% (20/23) and CR rate at 6 months is 22% (5/23) confirming results observed in the first cohort. One clonal evolution event (deletion 7p) occurred at 6 months in a responder and relapse of pancytopenia soon followed.
Summary
The addition of eltrombopag to IST increases overall and complete hematologic response rates. Marked increase in CD34+ cells, the appearance of multipotent progenitor cells, and rapid blood count recovery support a mechanism of action of direct stimulation of stem and progenitor cells by eltrombopag.
Keyword(s): Aplastic anemia, Bone marrow failure, Immune therapy, Stem and progenitor cell
Session topic: Biology and clinics of bone marrow failure syndromes and PNH
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:15 to 14.06.2015 08:30
Location: Room Stolz 2
Background
Immunosuppressive treatment (IST) for severe aplastic anemia (SAA) has a 60-65% rate of overall hematologic response (OR), of which only about 10% are complete (CR). Eltrombopag, a synthetic thrombopoietin receptor agonist (TPO-RA), has activity in refractory SAA, producing multilineage hematologic responses in about 40% of patients.
Aims
We conducted an investigator-initiated phase II, single-center trial to test the efficacy of adding eltrombopag to h-ATG/CsA (clinicaltrials.gov, NCT01623167).
Methods
Patients with treatment-naïve SAA were enrolled from July 2012 to November 2014. All subjects received standard h-ATG and CsA. To determine the optimum regimen, eltrombopag was administered at 150 mg daily to two consecutively enrolling cohorts (see Table). The primary endpoint was CR at 6 months. Both cohorts were powered to detect a ≥ 30% CR rate at 6 months for comparison with our historical rate of 10%. Our historical data suggest that CR is a surrogate for late events since it is associated with a low rate of evolution to monosomy 7/acute leukemia and correlates with excellent long-term survival.
Results
Thirty subjects enrolled in the first cohort had a median age of 39 years (12-72); thirteen (43%) had very severe disease (ANC<200/µl), and 8 (27%) had PNH clones ≥1%. Eltrombopag was well tolerated when combined with CsA, and only three patients discontinued treatment before 6 months. CR at 6 months was 33% (95% CI, 15-51%), significantly higher than our historical rate of 10% (p<0.001). OR at 6 months was 80% (CI, 65-95%), exceeding the historical rate of 60% (p=0.040). Median increases in blood counts among responders at 6 months (n=24) were: absolute neutrophil count (ANC) +1240/µl; hemoglobin +3.8 gm/dL; and platelets +87,000/µl. Median time to achieve transfusion independence was 32 days for platelets and 39 days for red cells. Serial BM biopsies showed improved cellularity in 25 cases without increased fibrosis. Median increase in BM CD34+ cells, as measured by flow cytometry, was 34-fold from baseline to 3 months, and 23-fold from baseline to 6 months (p<0.0001). Primitive hematopoietic progenitors were serially measured in 4 patients, detecting HSC (CD34+CD38-CD45RA-CD90+CD49f+Rholo) and MPP (CD34+CD38-CD45RA-CD90-CD49f-) by flow cytometry. Undetectable at baseline, these cells constituted 4-48 HSC and 1-27 MPP per 100,000 CD34+ cells 6 months after therapy. The first cohort has been followed for median 24 months (range 15-31m); there have been 3 clonal evolution events, all detected in responders: one patient had complex cytogenetics (t(3;3)(q21;q26)) with BM dysplasia followed by increased blasts, and deletion 13q in one subject and trisomy 15 in another, neither with BM dysplasia. Enrollment to the second cohort, in which eltrombopag is stopped at 3 months, is complete and 23 subjects are currently evaluable at 6 months. OR at 6 months is 87% (20/23) and CR rate at 6 months is 22% (5/23) confirming results observed in the first cohort. One clonal evolution event (deletion 7p) occurred at 6 months in a responder and relapse of pancytopenia soon followed.
Summary
The addition of eltrombopag to IST increases overall and complete hematologic response rates. Marked increase in CD34+ cells, the appearance of multipotent progenitor cells, and rapid blood count recovery support a mechanism of action of direct stimulation of stem and progenitor cells by eltrombopag.
Keyword(s): Aplastic anemia, Bone marrow failure, Immune therapy, Stem and progenitor cell

Session topic: Biology and clinics of bone marrow failure syndromes and PNH
Abstract: S826
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:15 to 14.06.2015 08:30
Location: Room Stolz 2
Background
Immunosuppressive treatment (IST) for severe aplastic anemia (SAA) has a 60-65% rate of overall hematologic response (OR), of which only about 10% are complete (CR). Eltrombopag, a synthetic thrombopoietin receptor agonist (TPO-RA), has activity in refractory SAA, producing multilineage hematologic responses in about 40% of patients.
Aims
We conducted an investigator-initiated phase II, single-center trial to test the efficacy of adding eltrombopag to h-ATG/CsA (clinicaltrials.gov, NCT01623167).
Methods
Patients with treatment-naïve SAA were enrolled from July 2012 to November 2014. All subjects received standard h-ATG and CsA. To determine the optimum regimen, eltrombopag was administered at 150 mg daily to two consecutively enrolling cohorts (see Table). The primary endpoint was CR at 6 months. Both cohorts were powered to detect a ≥ 30% CR rate at 6 months for comparison with our historical rate of 10%. Our historical data suggest that CR is a surrogate for late events since it is associated with a low rate of evolution to monosomy 7/acute leukemia and correlates with excellent long-term survival.
Results
Thirty subjects enrolled in the first cohort had a median age of 39 years (12-72); thirteen (43%) had very severe disease (ANC<200/µl), and 8 (27%) had PNH clones ≥1%. Eltrombopag was well tolerated when combined with CsA, and only three patients discontinued treatment before 6 months. CR at 6 months was 33% (95% CI, 15-51%), significantly higher than our historical rate of 10% (p<0.001). OR at 6 months was 80% (CI, 65-95%), exceeding the historical rate of 60% (p=0.040). Median increases in blood counts among responders at 6 months (n=24) were: absolute neutrophil count (ANC) +1240/µl; hemoglobin +3.8 gm/dL; and platelets +87,000/µl. Median time to achieve transfusion independence was 32 days for platelets and 39 days for red cells. Serial BM biopsies showed improved cellularity in 25 cases without increased fibrosis. Median increase in BM CD34+ cells, as measured by flow cytometry, was 34-fold from baseline to 3 months, and 23-fold from baseline to 6 months (p<0.0001). Primitive hematopoietic progenitors were serially measured in 4 patients, detecting HSC (CD34+CD38-CD45RA-CD90+CD49f+Rholo) and MPP (CD34+CD38-CD45RA-CD90-CD49f-) by flow cytometry. Undetectable at baseline, these cells constituted 4-48 HSC and 1-27 MPP per 100,000 CD34+ cells 6 months after therapy. The first cohort has been followed for median 24 months (range 15-31m); there have been 3 clonal evolution events, all detected in responders: one patient had complex cytogenetics (t(3;3)(q21;q26)) with BM dysplasia followed by increased blasts, and deletion 13q in one subject and trisomy 15 in another, neither with BM dysplasia. Enrollment to the second cohort, in which eltrombopag is stopped at 3 months, is complete and 23 subjects are currently evaluable at 6 months. OR at 6 months is 87% (20/23) and CR rate at 6 months is 22% (5/23) confirming results observed in the first cohort. One clonal evolution event (deletion 7p) occurred at 6 months in a responder and relapse of pancytopenia soon followed.
Summary
The addition of eltrombopag to IST increases overall and complete hematologic response rates. Marked increase in CD34+ cells, the appearance of multipotent progenitor cells, and rapid blood count recovery support a mechanism of action of direct stimulation of stem and progenitor cells by eltrombopag.
Keyword(s): Aplastic anemia, Bone marrow failure, Immune therapy, Stem and progenitor cell

Session topic: Biology and clinics of bone marrow failure syndromes and PNH
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:15 to 14.06.2015 08:30
Location: Room Stolz 2
Background
Immunosuppressive treatment (IST) for severe aplastic anemia (SAA) has a 60-65% rate of overall hematologic response (OR), of which only about 10% are complete (CR). Eltrombopag, a synthetic thrombopoietin receptor agonist (TPO-RA), has activity in refractory SAA, producing multilineage hematologic responses in about 40% of patients.
Aims
We conducted an investigator-initiated phase II, single-center trial to test the efficacy of adding eltrombopag to h-ATG/CsA (clinicaltrials.gov, NCT01623167).
Methods
Patients with treatment-naïve SAA were enrolled from July 2012 to November 2014. All subjects received standard h-ATG and CsA. To determine the optimum regimen, eltrombopag was administered at 150 mg daily to two consecutively enrolling cohorts (see Table). The primary endpoint was CR at 6 months. Both cohorts were powered to detect a ≥ 30% CR rate at 6 months for comparison with our historical rate of 10%. Our historical data suggest that CR is a surrogate for late events since it is associated with a low rate of evolution to monosomy 7/acute leukemia and correlates with excellent long-term survival.
Results
Thirty subjects enrolled in the first cohort had a median age of 39 years (12-72); thirteen (43%) had very severe disease (ANC<200/µl), and 8 (27%) had PNH clones ≥1%. Eltrombopag was well tolerated when combined with CsA, and only three patients discontinued treatment before 6 months. CR at 6 months was 33% (95% CI, 15-51%), significantly higher than our historical rate of 10% (p<0.001). OR at 6 months was 80% (CI, 65-95%), exceeding the historical rate of 60% (p=0.040). Median increases in blood counts among responders at 6 months (n=24) were: absolute neutrophil count (ANC) +1240/µl; hemoglobin +3.8 gm/dL; and platelets +87,000/µl. Median time to achieve transfusion independence was 32 days for platelets and 39 days for red cells. Serial BM biopsies showed improved cellularity in 25 cases without increased fibrosis. Median increase in BM CD34+ cells, as measured by flow cytometry, was 34-fold from baseline to 3 months, and 23-fold from baseline to 6 months (p<0.0001). Primitive hematopoietic progenitors were serially measured in 4 patients, detecting HSC (CD34+CD38-CD45RA-CD90+CD49f+Rholo) and MPP (CD34+CD38-CD45RA-CD90-CD49f-) by flow cytometry. Undetectable at baseline, these cells constituted 4-48 HSC and 1-27 MPP per 100,000 CD34+ cells 6 months after therapy. The first cohort has been followed for median 24 months (range 15-31m); there have been 3 clonal evolution events, all detected in responders: one patient had complex cytogenetics (t(3;3)(q21;q26)) with BM dysplasia followed by increased blasts, and deletion 13q in one subject and trisomy 15 in another, neither with BM dysplasia. Enrollment to the second cohort, in which eltrombopag is stopped at 3 months, is complete and 23 subjects are currently evaluable at 6 months. OR at 6 months is 87% (20/23) and CR rate at 6 months is 22% (5/23) confirming results observed in the first cohort. One clonal evolution event (deletion 7p) occurred at 6 months in a responder and relapse of pancytopenia soon followed.
Summary
The addition of eltrombopag to IST increases overall and complete hematologic response rates. Marked increase in CD34+ cells, the appearance of multipotent progenitor cells, and rapid blood count recovery support a mechanism of action of direct stimulation of stem and progenitor cells by eltrombopag.
Keyword(s): Aplastic anemia, Bone marrow failure, Immune therapy, Stem and progenitor cell

Session topic: Biology and clinics of bone marrow failure syndromes and PNH
{{ help_message }}
{{filter}}


