IMMUNOMODULATORY EFFECT OF VITAMIN D AFTER ALLOGENEIC STEM CELL TRANSPLANTATION (ALLOSCT): RESULTS OF A PROSPECTIVE MULTICENTER CLINICALTRIAL. ALOVITA; EUDRACT: 2010-023279-25
(Abstract release date: 05/21/15)
EHA Library. Antonio Perez-Simon J. 06/14/15; 103170; S801

Dr. Jose Antonio Perez-Simon
Contributions
Contributions
Abstract
Abstract: S801
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:15 to 14.06.2015 08:30
Location: Room Lehar 1 + 2
Background
Vitamin D receptor (VDR) is expressed on activated immune cells. Numerous preclinical studies, including models for solid organ transplantation, have shown that vitamin D (VitD) has a potent immunomodulatory effect although, by contrast, few reports have described an increased immune response upon exposure to vitD. In the alloSCT setting scanty information is available although an association between common polymorphisms of VDR and graft-versus-host disease (GVHD) has been described. In addition, different studies have also described the capability of vitD to induce differentiation of blasts in acute leukemia. In spite of these properties no clinical trial has been carried out to evaluate the role of VitD as an immunomodulatory agent after alloSCT.
Aims
Based on the potential benefit of vitD on the risk of GVHD but also considering previous studies describing an exacerbated immune response after exposure to the drug, the main aim was to determine the safety and to assess the effect of VitD supplementation after alloSCT on the incidence of GVHD.
Methods
We designed a multicenter prospective Phase I/II clinical trial with three consecutive cohorts of patients (n= 50 each group), receiving no vitD (control group, CG), 1000 IU/day po. VitD (low dose group, LDG) or 5000 IU/day (high-dose group, GDA) from day -5 to +100 post alloSCT. In addition, we measured plasma levels of VitD (on days -5, +1, +7, +14 and +21 after transplant), of serum th1/th2 cytokines (on days +1, +7, +14, +21, +56 and +100) and of WBC subpopulations including T cells (naive/memory/effector), activation markers for T cells, Treg, NK cells, B cells and dendritic cells by flow cytometry (on days +21, +56 and +100).
Results
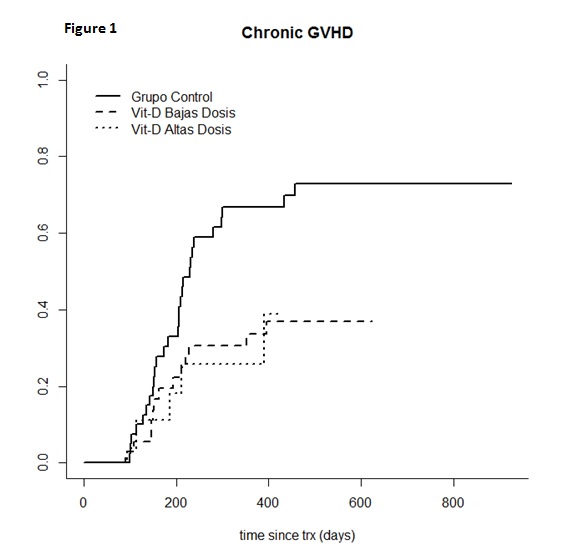
Regarding characteristics of patients, we only observed significant differences between the 3 subgroups in terms of age, patients in LDG being slightly older. Calcineurin inhibitors plus methotrexate or MMF and FK506 plus rapamycin were used as GVHD prophylaxis. No T-cell depletion was allowed as GVHD prophylaxis. No significant differences were observed in terms of cumulative incidence of global and grade II-IV of acute GVHD (CG= 55.9% and 38.2%; LDG= 56.8% and 36.4% and HDG= 56.4% and 53.8%, respectively). By contrast, a significantly lower cumulative incidence of chronic GVHD (cGVHD) at 1 year was observed in LDG and HDG as compared to patients who did not receive VitD (33.7%, 25.9% and 66.7% for LDG, HDG and CG, respectively; p=0.04) (Figure 1). Multivariate analysis identified treatment with VitD as the only variable which significantly decreased the risk of cGVHD (p=0.03) (for LDG [HR=0.33, (95% CI=0.13-0.81), p=0.01] and for HDG [HR=0.42, (95% CI=0.16-1.1), p=0.07]. No significant differences were observed in terms of relapse or non-relapse mortality. With a median follow up of 1 year, overall survival was 70,6%, 74.2% and 70.4% for CG, LDG and HDG, respectively, p=0.76). Concerning the biological parameters monitored, plasma levels of vitD were higher among patients receiving the drug as compared to the control group, beyond day +21 (significantly for HDG). VitD modified the immune response after alloSCT for parameters evaluated, the most significant differences being a decreased percentage of B-cells for both LDG and HDG (p≤0.05), a markedly modified ratio of naïve/central memory/effector T cells, with a lower number of circulating naïve CD8 T cells for patients receiving vitD (10.1%; 9.61%; 16%, p=0.02 for LGC, HGD and CG respectively, p≤0.025) and a lower expression of CD40L as marker of activation upon exposure to vitD (24.4%, 39.5% and 49%, for LGD, HGD and CG, respectively, p≤0.02).
Summary
Keyword(s): Acute graft-versus-host disease, Chronic graft-versus-host, Immunomodulation, Transplant
Session topic: Stem cell transplantation: Clinical 3
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:15 to 14.06.2015 08:30
Location: Room Lehar 1 + 2
Background
Vitamin D receptor (VDR) is expressed on activated immune cells. Numerous preclinical studies, including models for solid organ transplantation, have shown that vitamin D (VitD) has a potent immunomodulatory effect although, by contrast, few reports have described an increased immune response upon exposure to vitD. In the alloSCT setting scanty information is available although an association between common polymorphisms of VDR and graft-versus-host disease (GVHD) has been described. In addition, different studies have also described the capability of vitD to induce differentiation of blasts in acute leukemia. In spite of these properties no clinical trial has been carried out to evaluate the role of VitD as an immunomodulatory agent after alloSCT.
Aims
Based on the potential benefit of vitD on the risk of GVHD but also considering previous studies describing an exacerbated immune response after exposure to the drug, the main aim was to determine the safety and to assess the effect of VitD supplementation after alloSCT on the incidence of GVHD.
Methods
We designed a multicenter prospective Phase I/II clinical trial with three consecutive cohorts of patients (n= 50 each group), receiving no vitD (control group, CG), 1000 IU/day po. VitD (low dose group, LDG) or 5000 IU/day (high-dose group, GDA) from day -5 to +100 post alloSCT. In addition, we measured plasma levels of VitD (on days -5, +1, +7, +14 and +21 after transplant), of serum th1/th2 cytokines (on days +1, +7, +14, +21, +56 and +100) and of WBC subpopulations including T cells (naive/memory/effector), activation markers for T cells, Treg, NK cells, B cells and dendritic cells by flow cytometry (on days +21, +56 and +100).
Results
Regarding characteristics of patients, we only observed significant differences between the 3 subgroups in terms of age, patients in LDG being slightly older. Calcineurin inhibitors plus methotrexate or MMF and FK506 plus rapamycin were used as GVHD prophylaxis. No T-cell depletion was allowed as GVHD prophylaxis. No significant differences were observed in terms of cumulative incidence of global and grade II-IV of acute GVHD (CG= 55.9% and 38.2%; LDG= 56.8% and 36.4% and HDG= 56.4% and 53.8%, respectively). By contrast, a significantly lower cumulative incidence of chronic GVHD (cGVHD) at 1 year was observed in LDG and HDG as compared to patients who did not receive VitD (33.7%, 25.9% and 66.7% for LDG, HDG and CG, respectively; p=0.04) (Figure 1). Multivariate analysis identified treatment with VitD as the only variable which significantly decreased the risk of cGVHD (p=0.03) (for LDG [HR=0.33, (95% CI=0.13-0.81), p=0.01] and for HDG [HR=0.42, (95% CI=0.16-1.1), p=0.07]. No significant differences were observed in terms of relapse or non-relapse mortality. With a median follow up of 1 year, overall survival was 70,6%, 74.2% and 70.4% for CG, LDG and HDG, respectively, p=0.76). Concerning the biological parameters monitored, plasma levels of vitD were higher among patients receiving the drug as compared to the control group, beyond day +21 (significantly for HDG). VitD modified the immune response after alloSCT for parameters evaluated, the most significant differences being a decreased percentage of B-cells for both LDG and HDG (p≤0.05), a markedly modified ratio of naïve/central memory/effector T cells, with a lower number of circulating naïve CD8 T cells for patients receiving vitD (10.1%; 9.61%; 16%, p=0.02 for LGC, HGD and CG respectively, p≤0.025) and a lower expression of CD40L as marker of activation upon exposure to vitD (24.4%, 39.5% and 49%, for LGD, HGD and CG, respectively, p≤0.02).
Summary
This is the first prospective multicenter trial which analyzes the effect of vitamin D administration after alloSCT. A significantly lower incidence of cGVHD was observed among patients receiving vitD. Interestingly, vitD markedly modified the immune response after alloSCT.
Keyword(s): Acute graft-versus-host disease, Chronic graft-versus-host, Immunomodulation, Transplant

Session topic: Stem cell transplantation: Clinical 3
Abstract: S801
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:15 to 14.06.2015 08:30
Location: Room Lehar 1 + 2
Background
Vitamin D receptor (VDR) is expressed on activated immune cells. Numerous preclinical studies, including models for solid organ transplantation, have shown that vitamin D (VitD) has a potent immunomodulatory effect although, by contrast, few reports have described an increased immune response upon exposure to vitD. In the alloSCT setting scanty information is available although an association between common polymorphisms of VDR and graft-versus-host disease (GVHD) has been described. In addition, different studies have also described the capability of vitD to induce differentiation of blasts in acute leukemia. In spite of these properties no clinical trial has been carried out to evaluate the role of VitD as an immunomodulatory agent after alloSCT.
Aims
Based on the potential benefit of vitD on the risk of GVHD but also considering previous studies describing an exacerbated immune response after exposure to the drug, the main aim was to determine the safety and to assess the effect of VitD supplementation after alloSCT on the incidence of GVHD.
Methods
We designed a multicenter prospective Phase I/II clinical trial with three consecutive cohorts of patients (n= 50 each group), receiving no vitD (control group, CG), 1000 IU/day po. VitD (low dose group, LDG) or 5000 IU/day (high-dose group, GDA) from day -5 to +100 post alloSCT. In addition, we measured plasma levels of VitD (on days -5, +1, +7, +14 and +21 after transplant), of serum th1/th2 cytokines (on days +1, +7, +14, +21, +56 and +100) and of WBC subpopulations including T cells (naive/memory/effector), activation markers for T cells, Treg, NK cells, B cells and dendritic cells by flow cytometry (on days +21, +56 and +100).
Results
Regarding characteristics of patients, we only observed significant differences between the 3 subgroups in terms of age, patients in LDG being slightly older. Calcineurin inhibitors plus methotrexate or MMF and FK506 plus rapamycin were used as GVHD prophylaxis. No T-cell depletion was allowed as GVHD prophylaxis. No significant differences were observed in terms of cumulative incidence of global and grade II-IV of acute GVHD (CG= 55.9% and 38.2%; LDG= 56.8% and 36.4% and HDG= 56.4% and 53.8%, respectively). By contrast, a significantly lower cumulative incidence of chronic GVHD (cGVHD) at 1 year was observed in LDG and HDG as compared to patients who did not receive VitD (33.7%, 25.9% and 66.7% for LDG, HDG and CG, respectively; p=0.04) (Figure 1). Multivariate analysis identified treatment with VitD as the only variable which significantly decreased the risk of cGVHD (p=0.03) (for LDG [HR=0.33, (95% CI=0.13-0.81), p=0.01] and for HDG [HR=0.42, (95% CI=0.16-1.1), p=0.07]. No significant differences were observed in terms of relapse or non-relapse mortality. With a median follow up of 1 year, overall survival was 70,6%, 74.2% and 70.4% for CG, LDG and HDG, respectively, p=0.76). Concerning the biological parameters monitored, plasma levels of vitD were higher among patients receiving the drug as compared to the control group, beyond day +21 (significantly for HDG). VitD modified the immune response after alloSCT for parameters evaluated, the most significant differences being a decreased percentage of B-cells for both LDG and HDG (p≤0.05), a markedly modified ratio of naïve/central memory/effector T cells, with a lower number of circulating naïve CD8 T cells for patients receiving vitD (10.1%; 9.61%; 16%, p=0.02 for LGC, HGD and CG respectively, p≤0.025) and a lower expression of CD40L as marker of activation upon exposure to vitD (24.4%, 39.5% and 49%, for LGD, HGD and CG, respectively, p≤0.02).
Summary
Keyword(s): Acute graft-versus-host disease, Chronic graft-versus-host, Immunomodulation, Transplant

Session topic: Stem cell transplantation: Clinical 3
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:15 to 14.06.2015 08:30
Location: Room Lehar 1 + 2
Background
Vitamin D receptor (VDR) is expressed on activated immune cells. Numerous preclinical studies, including models for solid organ transplantation, have shown that vitamin D (VitD) has a potent immunomodulatory effect although, by contrast, few reports have described an increased immune response upon exposure to vitD. In the alloSCT setting scanty information is available although an association between common polymorphisms of VDR and graft-versus-host disease (GVHD) has been described. In addition, different studies have also described the capability of vitD to induce differentiation of blasts in acute leukemia. In spite of these properties no clinical trial has been carried out to evaluate the role of VitD as an immunomodulatory agent after alloSCT.
Aims
Based on the potential benefit of vitD on the risk of GVHD but also considering previous studies describing an exacerbated immune response after exposure to the drug, the main aim was to determine the safety and to assess the effect of VitD supplementation after alloSCT on the incidence of GVHD.
Methods
We designed a multicenter prospective Phase I/II clinical trial with three consecutive cohorts of patients (n= 50 each group), receiving no vitD (control group, CG), 1000 IU/day po. VitD (low dose group, LDG) or 5000 IU/day (high-dose group, GDA) from day -5 to +100 post alloSCT. In addition, we measured plasma levels of VitD (on days -5, +1, +7, +14 and +21 after transplant), of serum th1/th2 cytokines (on days +1, +7, +14, +21, +56 and +100) and of WBC subpopulations including T cells (naive/memory/effector), activation markers for T cells, Treg, NK cells, B cells and dendritic cells by flow cytometry (on days +21, +56 and +100).
Results
Regarding characteristics of patients, we only observed significant differences between the 3 subgroups in terms of age, patients in LDG being slightly older. Calcineurin inhibitors plus methotrexate or MMF and FK506 plus rapamycin were used as GVHD prophylaxis. No T-cell depletion was allowed as GVHD prophylaxis. No significant differences were observed in terms of cumulative incidence of global and grade II-IV of acute GVHD (CG= 55.9% and 38.2%; LDG= 56.8% and 36.4% and HDG= 56.4% and 53.8%, respectively). By contrast, a significantly lower cumulative incidence of chronic GVHD (cGVHD) at 1 year was observed in LDG and HDG as compared to patients who did not receive VitD (33.7%, 25.9% and 66.7% for LDG, HDG and CG, respectively; p=0.04) (Figure 1). Multivariate analysis identified treatment with VitD as the only variable which significantly decreased the risk of cGVHD (p=0.03) (for LDG [HR=0.33, (95% CI=0.13-0.81), p=0.01] and for HDG [HR=0.42, (95% CI=0.16-1.1), p=0.07]. No significant differences were observed in terms of relapse or non-relapse mortality. With a median follow up of 1 year, overall survival was 70,6%, 74.2% and 70.4% for CG, LDG and HDG, respectively, p=0.76). Concerning the biological parameters monitored, plasma levels of vitD were higher among patients receiving the drug as compared to the control group, beyond day +21 (significantly for HDG). VitD modified the immune response after alloSCT for parameters evaluated, the most significant differences being a decreased percentage of B-cells for both LDG and HDG (p≤0.05), a markedly modified ratio of naïve/central memory/effector T cells, with a lower number of circulating naïve CD8 T cells for patients receiving vitD (10.1%; 9.61%; 16%, p=0.02 for LGC, HGD and CG respectively, p≤0.025) and a lower expression of CD40L as marker of activation upon exposure to vitD (24.4%, 39.5% and 49%, for LGD, HGD and CG, respectively, p≤0.02).
Summary
This is the first prospective multicenter trial which analyzes the effect of vitamin D administration after alloSCT. A significantly lower incidence of cGVHD was observed among patients receiving vitD. Interestingly, vitD markedly modified the immune response after alloSCT.
Keyword(s): Acute graft-versus-host disease, Chronic graft-versus-host, Immunomodulation, Transplant

Session topic: Stem cell transplantation: Clinical 3
{{ help_message }}
{{filter}}


