IMMUNOSUPPRESSION BY MESENCHYMAL STROMAL CELLS DERIVED FROM HUMAN INDUCED PLURIPOTENT STEM CELLS : EVALUATION IN AN AGVHD MODEL
(Abstract release date: 05/21/15)
EHA Library. Roux C. 06/13/15; 103167; S494
Disclosure(s): ARCHET CHU NICEhématologie pédiatrie

Clémence Roux
Contributions
Contributions
Abstract
Abstract: S494
Type: Oral Presentation + travel grant
Presentation during EHA20: From 13.06.2015 16:30 to 13.06.2015 16:45
Location: Room Lehar 3 + 4
Background
One major problem of allogeneic hematopoietic stem cell transplantation (HSCT) is acute Graft versus Host Disease (aGVHD). aGVHD has been managed until now with HLA matching and a constant evolving repertoire of immunosuppressive drugs. One alternative would be to generate in the host a permanent tolerance state toward the graft. Tolerant inducing cell therapy has been proposed with adult mesenchymal stromal cells (MSCs). Ex vivo isolated somatic MSCs have been implicated in immunoregulatory functions on cells from both the innate and adaptive immune system. They were proposed for cell therapies for treatment of aGVHD, Nevertheless, their use is restricted because of the few number that can be recovered from adult tissues, their limited in vitro expansion, and the absence of a full phenotypic characterization. Therefore other sources of well-defined and unlimited number of MSCs are needed, and MSCs derived in vitro from human Induced pluripotent stem cell (huIPS) would be a valuable tool for therapeutic approaches.
Aims
Because of our expertise in pluripotent stem cell differentiation, we generated huiPS-MSCs that present a strong immunosuppressive activity on allogeneic T cell responses. Our objectives are: 1/ To evaluate and characterize in vitro this immunosuppression. 2/ To validate in vivo these results using a xenoGVHD model.
Methods
To characterize the huIPS-MSCs in vitro, FACS phenotyping and multipotency were tested. Their immunogenicity in vitro was monitored in co-cultures with allogenic peripheral blood mononuclear cells (PBMC). The in vivo immunosuppressive activity of huiPS-MSCs was evaluated using a xenoGVHD model in immunodeficient NSG (NOD/SCID/IL2rγKO) mice in which human PBMC were injected intra-peritonally. We established 3 groups : 1) huIPS-MSCs (control) 2) PBMC 3) PBMC + IPS-MSCs. We repeated huIPS-MSCs injection weekly with median number of injection n=3 (range 2-3). The activation state of human allogeneic T lymphocytes recovered from mice between 5 to 8 weeks after initial injection was evaluated and indicated the level of the xenoGVHD process and the efficiency of huiPS-MSCs to prevent it.
Results
a) In vitro characterization of huIPS-MSCs
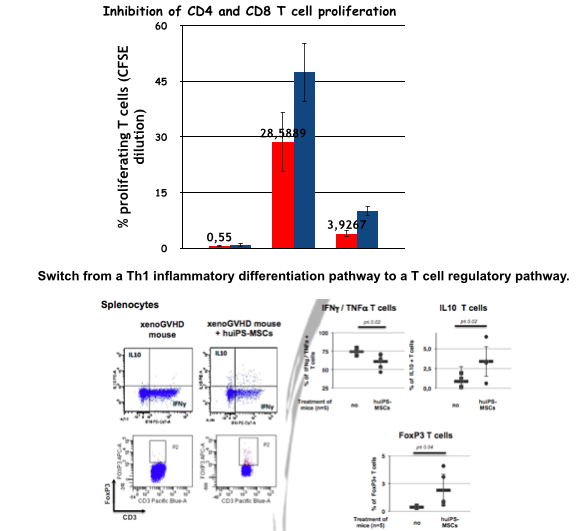
As expected, the huiPS-MSCs were positive for CD73, CD90, CD105, HLA-I Ags and negative for CD45, CD34, HLA-II Ags and they were capable of differentiation into the classical mesenchymal-derived cells (osteoblast, chondrocytes and adipocytes). To test their immunosuppressive properties, we analyzed their action on the proliferation of human T lymphocytes stimulated in an allogeneic manner (Fig 1). The stimulation of PBMC in mixed lymphocyte reaction resulted in CD4 and CD8 T cell proliferation (28 ± 7% and 47 ± 8%, respectively), which was significantly reduced in co-culture with huiPS-MSCs (4 ± 2% and 10± 2 respectively, n=3 p<0,05). We were able to demonstrate using blocking antibodies that part of the inhibition exerted by the iPS-MSCs is due to a) B7H1, a membrane receptor for the B7 family, known for its inhibitory action on the activation of T lymphocytes b) and B7H3 (from the same family) whose role remains controversial.
b) In vivo characterization of huIPS-MSCs
After sacrifice of mice, human circulating cells, those present in the peritoneal cavity and in the spleen were analysed by FACS. Mostly T lymphocytes were detected, and their number was significantly reduced in mice treated with huIPS-MSCs p<0,05.
Intracytoplasmic labelling of recovered T cells showed that untreated mice displayed high percentages of human differentiated T cells producing IFN g and TNF a (typical of a inflammatory Th1 cytokine polarization profile), while little or none produced low inflammatory (IL-4) or anti-inflammatory (IL-10) cytokines. In contrast, in mice treated with the huiPS-MSCs, the proportion of T cells of the Th1 type was substantially reduced, while that of T cells producing IL-4 and / or IL-10 was slightly increased. In parallel, T cells expressing FoxP3 appeared (Fig 1).
Summary
We were able to generate immune-modulatory huiPS-MSCs that can be used to reduce activation of T cells in a xeno-aGVHD model through a switch from a Th1 inflammatory differentiation pathway to a T cell regulatory pathway. Our results may favor the development of new tools and strategies based on the use of pluripotent stem cells and their derivatives to prevent aGVHD but also for the induction of specific tolerance.
Keyword(s): Acute graft-versus-host disease, Immunosuppression, Mesenchymal stem cell, Stem cell transplant
Session topic: Stem cell transplantation: Experimental
Type: Oral Presentation + travel grant
Presentation during EHA20: From 13.06.2015 16:30 to 13.06.2015 16:45
Location: Room Lehar 3 + 4
Background
One major problem of allogeneic hematopoietic stem cell transplantation (HSCT) is acute Graft versus Host Disease (aGVHD). aGVHD has been managed until now with HLA matching and a constant evolving repertoire of immunosuppressive drugs. One alternative would be to generate in the host a permanent tolerance state toward the graft. Tolerant inducing cell therapy has been proposed with adult mesenchymal stromal cells (MSCs). Ex vivo isolated somatic MSCs have been implicated in immunoregulatory functions on cells from both the innate and adaptive immune system. They were proposed for cell therapies for treatment of aGVHD, Nevertheless, their use is restricted because of the few number that can be recovered from adult tissues, their limited in vitro expansion, and the absence of a full phenotypic characterization. Therefore other sources of well-defined and unlimited number of MSCs are needed, and MSCs derived in vitro from human Induced pluripotent stem cell (huIPS) would be a valuable tool for therapeutic approaches.
Aims
Because of our expertise in pluripotent stem cell differentiation, we generated huiPS-MSCs that present a strong immunosuppressive activity on allogeneic T cell responses. Our objectives are: 1/ To evaluate and characterize in vitro this immunosuppression. 2/ To validate in vivo these results using a xenoGVHD model.
Methods
To characterize the huIPS-MSCs in vitro, FACS phenotyping and multipotency were tested. Their immunogenicity in vitro was monitored in co-cultures with allogenic peripheral blood mononuclear cells (PBMC). The in vivo immunosuppressive activity of huiPS-MSCs was evaluated using a xenoGVHD model in immunodeficient NSG (NOD/SCID/IL2rγKO) mice in which human PBMC were injected intra-peritonally. We established 3 groups : 1) huIPS-MSCs (control) 2) PBMC 3) PBMC + IPS-MSCs. We repeated huIPS-MSCs injection weekly with median number of injection n=3 (range 2-3). The activation state of human allogeneic T lymphocytes recovered from mice between 5 to 8 weeks after initial injection was evaluated and indicated the level of the xenoGVHD process and the efficiency of huiPS-MSCs to prevent it.
Results
a) In vitro characterization of huIPS-MSCs
As expected, the huiPS-MSCs were positive for CD73, CD90, CD105, HLA-I Ags and negative for CD45, CD34, HLA-II Ags and they were capable of differentiation into the classical mesenchymal-derived cells (osteoblast, chondrocytes and adipocytes). To test their immunosuppressive properties, we analyzed their action on the proliferation of human T lymphocytes stimulated in an allogeneic manner (Fig 1). The stimulation of PBMC in mixed lymphocyte reaction resulted in CD4 and CD8 T cell proliferation (28 ± 7% and 47 ± 8%, respectively), which was significantly reduced in co-culture with huiPS-MSCs (4 ± 2% and 10± 2 respectively, n=3 p<0,05). We were able to demonstrate using blocking antibodies that part of the inhibition exerted by the iPS-MSCs is due to a) B7H1, a membrane receptor for the B7 family, known for its inhibitory action on the activation of T lymphocytes b) and B7H3 (from the same family) whose role remains controversial.
b) In vivo characterization of huIPS-MSCs
After sacrifice of mice, human circulating cells, those present in the peritoneal cavity and in the spleen were analysed by FACS. Mostly T lymphocytes were detected, and their number was significantly reduced in mice treated with huIPS-MSCs p<0,05.
Intracytoplasmic labelling of recovered T cells showed that untreated mice displayed high percentages of human differentiated T cells producing IFN g and TNF a (typical of a inflammatory Th1 cytokine polarization profile), while little or none produced low inflammatory (IL-4) or anti-inflammatory (IL-10) cytokines. In contrast, in mice treated with the huiPS-MSCs, the proportion of T cells of the Th1 type was substantially reduced, while that of T cells producing IL-4 and / or IL-10 was slightly increased. In parallel, T cells expressing FoxP3 appeared (Fig 1).
Summary
We were able to generate immune-modulatory huiPS-MSCs that can be used to reduce activation of T cells in a xeno-aGVHD model through a switch from a Th1 inflammatory differentiation pathway to a T cell regulatory pathway. Our results may favor the development of new tools and strategies based on the use of pluripotent stem cells and their derivatives to prevent aGVHD but also for the induction of specific tolerance.
Keyword(s): Acute graft-versus-host disease, Immunosuppression, Mesenchymal stem cell, Stem cell transplant

Session topic: Stem cell transplantation: Experimental
Abstract: S494
Type: Oral Presentation + travel grant
Presentation during EHA20: From 13.06.2015 16:30 to 13.06.2015 16:45
Location: Room Lehar 3 + 4
Background
One major problem of allogeneic hematopoietic stem cell transplantation (HSCT) is acute Graft versus Host Disease (aGVHD). aGVHD has been managed until now with HLA matching and a constant evolving repertoire of immunosuppressive drugs. One alternative would be to generate in the host a permanent tolerance state toward the graft. Tolerant inducing cell therapy has been proposed with adult mesenchymal stromal cells (MSCs). Ex vivo isolated somatic MSCs have been implicated in immunoregulatory functions on cells from both the innate and adaptive immune system. They were proposed for cell therapies for treatment of aGVHD, Nevertheless, their use is restricted because of the few number that can be recovered from adult tissues, their limited in vitro expansion, and the absence of a full phenotypic characterization. Therefore other sources of well-defined and unlimited number of MSCs are needed, and MSCs derived in vitro from human Induced pluripotent stem cell (huIPS) would be a valuable tool for therapeutic approaches.
Aims
Because of our expertise in pluripotent stem cell differentiation, we generated huiPS-MSCs that present a strong immunosuppressive activity on allogeneic T cell responses. Our objectives are: 1/ To evaluate and characterize in vitro this immunosuppression. 2/ To validate in vivo these results using a xenoGVHD model.
Methods
To characterize the huIPS-MSCs in vitro, FACS phenotyping and multipotency were tested. Their immunogenicity in vitro was monitored in co-cultures with allogenic peripheral blood mononuclear cells (PBMC). The in vivo immunosuppressive activity of huiPS-MSCs was evaluated using a xenoGVHD model in immunodeficient NSG (NOD/SCID/IL2rγKO) mice in which human PBMC were injected intra-peritonally. We established 3 groups : 1) huIPS-MSCs (control) 2) PBMC 3) PBMC + IPS-MSCs. We repeated huIPS-MSCs injection weekly with median number of injection n=3 (range 2-3). The activation state of human allogeneic T lymphocytes recovered from mice between 5 to 8 weeks after initial injection was evaluated and indicated the level of the xenoGVHD process and the efficiency of huiPS-MSCs to prevent it.
Results
a) In vitro characterization of huIPS-MSCs
As expected, the huiPS-MSCs were positive for CD73, CD90, CD105, HLA-I Ags and negative for CD45, CD34, HLA-II Ags and they were capable of differentiation into the classical mesenchymal-derived cells (osteoblast, chondrocytes and adipocytes). To test their immunosuppressive properties, we analyzed their action on the proliferation of human T lymphocytes stimulated in an allogeneic manner (Fig 1). The stimulation of PBMC in mixed lymphocyte reaction resulted in CD4 and CD8 T cell proliferation (28 ± 7% and 47 ± 8%, respectively), which was significantly reduced in co-culture with huiPS-MSCs (4 ± 2% and 10± 2 respectively, n=3 p<0,05). We were able to demonstrate using blocking antibodies that part of the inhibition exerted by the iPS-MSCs is due to a) B7H1, a membrane receptor for the B7 family, known for its inhibitory action on the activation of T lymphocytes b) and B7H3 (from the same family) whose role remains controversial.
b) In vivo characterization of huIPS-MSCs
After sacrifice of mice, human circulating cells, those present in the peritoneal cavity and in the spleen were analysed by FACS. Mostly T lymphocytes were detected, and their number was significantly reduced in mice treated with huIPS-MSCs p<0,05.
Intracytoplasmic labelling of recovered T cells showed that untreated mice displayed high percentages of human differentiated T cells producing IFN g and TNF a (typical of a inflammatory Th1 cytokine polarization profile), while little or none produced low inflammatory (IL-4) or anti-inflammatory (IL-10) cytokines. In contrast, in mice treated with the huiPS-MSCs, the proportion of T cells of the Th1 type was substantially reduced, while that of T cells producing IL-4 and / or IL-10 was slightly increased. In parallel, T cells expressing FoxP3 appeared (Fig 1).
Summary
We were able to generate immune-modulatory huiPS-MSCs that can be used to reduce activation of T cells in a xeno-aGVHD model through a switch from a Th1 inflammatory differentiation pathway to a T cell regulatory pathway. Our results may favor the development of new tools and strategies based on the use of pluripotent stem cells and their derivatives to prevent aGVHD but also for the induction of specific tolerance.
Keyword(s): Acute graft-versus-host disease, Immunosuppression, Mesenchymal stem cell, Stem cell transplant

Session topic: Stem cell transplantation: Experimental
Type: Oral Presentation + travel grant
Presentation during EHA20: From 13.06.2015 16:30 to 13.06.2015 16:45
Location: Room Lehar 3 + 4
Background
One major problem of allogeneic hematopoietic stem cell transplantation (HSCT) is acute Graft versus Host Disease (aGVHD). aGVHD has been managed until now with HLA matching and a constant evolving repertoire of immunosuppressive drugs. One alternative would be to generate in the host a permanent tolerance state toward the graft. Tolerant inducing cell therapy has been proposed with adult mesenchymal stromal cells (MSCs). Ex vivo isolated somatic MSCs have been implicated in immunoregulatory functions on cells from both the innate and adaptive immune system. They were proposed for cell therapies for treatment of aGVHD, Nevertheless, their use is restricted because of the few number that can be recovered from adult tissues, their limited in vitro expansion, and the absence of a full phenotypic characterization. Therefore other sources of well-defined and unlimited number of MSCs are needed, and MSCs derived in vitro from human Induced pluripotent stem cell (huIPS) would be a valuable tool for therapeutic approaches.
Aims
Because of our expertise in pluripotent stem cell differentiation, we generated huiPS-MSCs that present a strong immunosuppressive activity on allogeneic T cell responses. Our objectives are: 1/ To evaluate and characterize in vitro this immunosuppression. 2/ To validate in vivo these results using a xenoGVHD model.
Methods
To characterize the huIPS-MSCs in vitro, FACS phenotyping and multipotency were tested. Their immunogenicity in vitro was monitored in co-cultures with allogenic peripheral blood mononuclear cells (PBMC). The in vivo immunosuppressive activity of huiPS-MSCs was evaluated using a xenoGVHD model in immunodeficient NSG (NOD/SCID/IL2rγKO) mice in which human PBMC were injected intra-peritonally. We established 3 groups : 1) huIPS-MSCs (control) 2) PBMC 3) PBMC + IPS-MSCs. We repeated huIPS-MSCs injection weekly with median number of injection n=3 (range 2-3). The activation state of human allogeneic T lymphocytes recovered from mice between 5 to 8 weeks after initial injection was evaluated and indicated the level of the xenoGVHD process and the efficiency of huiPS-MSCs to prevent it.
Results
a) In vitro characterization of huIPS-MSCs
As expected, the huiPS-MSCs were positive for CD73, CD90, CD105, HLA-I Ags and negative for CD45, CD34, HLA-II Ags and they were capable of differentiation into the classical mesenchymal-derived cells (osteoblast, chondrocytes and adipocytes). To test their immunosuppressive properties, we analyzed their action on the proliferation of human T lymphocytes stimulated in an allogeneic manner (Fig 1). The stimulation of PBMC in mixed lymphocyte reaction resulted in CD4 and CD8 T cell proliferation (28 ± 7% and 47 ± 8%, respectively), which was significantly reduced in co-culture with huiPS-MSCs (4 ± 2% and 10± 2 respectively, n=3 p<0,05). We were able to demonstrate using blocking antibodies that part of the inhibition exerted by the iPS-MSCs is due to a) B7H1, a membrane receptor for the B7 family, known for its inhibitory action on the activation of T lymphocytes b) and B7H3 (from the same family) whose role remains controversial.
b) In vivo characterization of huIPS-MSCs
After sacrifice of mice, human circulating cells, those present in the peritoneal cavity and in the spleen were analysed by FACS. Mostly T lymphocytes were detected, and their number was significantly reduced in mice treated with huIPS-MSCs p<0,05.
Intracytoplasmic labelling of recovered T cells showed that untreated mice displayed high percentages of human differentiated T cells producing IFN g and TNF a (typical of a inflammatory Th1 cytokine polarization profile), while little or none produced low inflammatory (IL-4) or anti-inflammatory (IL-10) cytokines. In contrast, in mice treated with the huiPS-MSCs, the proportion of T cells of the Th1 type was substantially reduced, while that of T cells producing IL-4 and / or IL-10 was slightly increased. In parallel, T cells expressing FoxP3 appeared (Fig 1).
Summary
We were able to generate immune-modulatory huiPS-MSCs that can be used to reduce activation of T cells in a xeno-aGVHD model through a switch from a Th1 inflammatory differentiation pathway to a T cell regulatory pathway. Our results may favor the development of new tools and strategies based on the use of pluripotent stem cells and their derivatives to prevent aGVHD but also for the induction of specific tolerance.
Keyword(s): Acute graft-versus-host disease, Immunosuppression, Mesenchymal stem cell, Stem cell transplant

Session topic: Stem cell transplantation: Experimental
{{ help_message }}
{{filter}}


