OVERALL SURVIVAL ANALYSIS ADJUSTING FOR TREATMENT EFFECT AFTER CROSSOVER IN A PHASE 3 STUDY EVALUATING IDELALISIB IN COMBINATION WITH RITUXIMAB IN RELAPSED CHRONIC LYMPHOCYTIC LEUKEMIA (CLL)
(Abstract release date: 05/21/15)
EHA Library. Ghia P. 06/14/15; 103166; S793

Dr. Paolo Ghia
Contributions
Contributions
Abstract
Abstract: S793
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:45 to 14.06.2015 09:00
Location: Room A7
Background
A Phase 3 trial evaluated idelalisib (IDELA) + rituximab (R) vs placebo (PBO) + R in patients (pts) with relapsed CLL (Furman, NEJM, 2014). Using an extension study design, pts on the control arm were allowed to receive IDELA after progression (PD). Estimating the true overall survival (OS) benefit of the active arm of randomized studies with such a crossover design represents a challenging statistical problem. Intent-to-treat (ITT) analyses considering data from pts after crossover as well as the approach of censoring subjects at the time point of crossover may significantly underestimate the OS benefit. Alternative analysis approaches like the rank-preserving structural failure time (RPSFT) model (Robins, 1991; Ishak, 2014) have successfully been used to estimate the true treatment effect in similar trials designs with crossover.
Aims
Estimate the treatment effect in OS adjusted for the impact of crossover
Methods
Three methods of OS analysis were compared (data cut-off date 01July2014): 1) ITT analysis calculating the OS according to initial randomization including data after crossover (as pre-specified), 2) Censoring subjects at the time point of crossover, and 3) RPSFT.
Methods 1 and 2 commonly underestimate the true OS benefit. The RPSFT model estimates the counterfactual event time of the control arm that would have been observed without crossover and provides the estimate of treatment effect adjusted for the impact of crossover.
Results
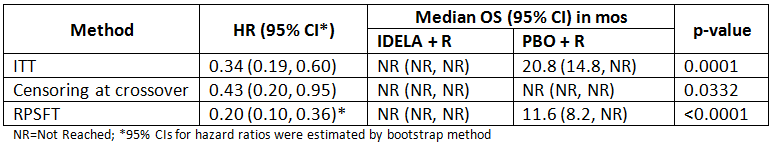
110 pts were randomized to each arm. 38% (n=42) of pts on PBO + R crossed over to receive single-agent IDELA following PD according to the initial study design. An additional 40% (n=44) of subjects on PBO + R crossed over to receive idelalisib in the absence of prior PD according to an amended protocol after the study was stopped due to overwhelming PFS efficacy. The median follow-up for the presented analyses was 12.5 months (mos) for pts initially on IDELA + R and 11.1 mos for PBO + R. There were 17 deaths in the IDELA + R group, and 40 deaths in the PBO + R group. Analysis results from each method are presented in the table below:
Summary
IDELA+R demonstrated significant and consistent improvement in OS regardless of the analysis method. The median OS and HR as estimated by the RPSFT model reduced the bias associated with the idelalisib treatment effect compared to the ITT analysis or the censoring approach.
Keyword(s): Chronic lymphocytic leukemia, Clinical outcome, Phase III, PI3K
Session topic: CLL: Refining outcomes
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:45 to 14.06.2015 09:00
Location: Room A7
Background
A Phase 3 trial evaluated idelalisib (IDELA) + rituximab (R) vs placebo (PBO) + R in patients (pts) with relapsed CLL (Furman, NEJM, 2014). Using an extension study design, pts on the control arm were allowed to receive IDELA after progression (PD). Estimating the true overall survival (OS) benefit of the active arm of randomized studies with such a crossover design represents a challenging statistical problem. Intent-to-treat (ITT) analyses considering data from pts after crossover as well as the approach of censoring subjects at the time point of crossover may significantly underestimate the OS benefit. Alternative analysis approaches like the rank-preserving structural failure time (RPSFT) model (Robins, 1991; Ishak, 2014) have successfully been used to estimate the true treatment effect in similar trials designs with crossover.
Aims
Estimate the treatment effect in OS adjusted for the impact of crossover
Methods
Three methods of OS analysis were compared (data cut-off date 01July2014): 1) ITT analysis calculating the OS according to initial randomization including data after crossover (as pre-specified), 2) Censoring subjects at the time point of crossover, and 3) RPSFT.
Methods 1 and 2 commonly underestimate the true OS benefit. The RPSFT model estimates the counterfactual event time of the control arm that would have been observed without crossover and provides the estimate of treatment effect adjusted for the impact of crossover.
Results
110 pts were randomized to each arm. 38% (n=42) of pts on PBO + R crossed over to receive single-agent IDELA following PD according to the initial study design. An additional 40% (n=44) of subjects on PBO + R crossed over to receive idelalisib in the absence of prior PD according to an amended protocol after the study was stopped due to overwhelming PFS efficacy. The median follow-up for the presented analyses was 12.5 months (mos) for pts initially on IDELA + R and 11.1 mos for PBO + R. There were 17 deaths in the IDELA + R group, and 40 deaths in the PBO + R group. Analysis results from each method are presented in the table below:
Summary
IDELA+R demonstrated significant and consistent improvement in OS regardless of the analysis method. The median OS and HR as estimated by the RPSFT model reduced the bias associated with the idelalisib treatment effect compared to the ITT analysis or the censoring approach.
Keyword(s): Chronic lymphocytic leukemia, Clinical outcome, Phase III, PI3K

Session topic: CLL: Refining outcomes
Abstract: S793
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:45 to 14.06.2015 09:00
Location: Room A7
Background
A Phase 3 trial evaluated idelalisib (IDELA) + rituximab (R) vs placebo (PBO) + R in patients (pts) with relapsed CLL (Furman, NEJM, 2014). Using an extension study design, pts on the control arm were allowed to receive IDELA after progression (PD). Estimating the true overall survival (OS) benefit of the active arm of randomized studies with such a crossover design represents a challenging statistical problem. Intent-to-treat (ITT) analyses considering data from pts after crossover as well as the approach of censoring subjects at the time point of crossover may significantly underestimate the OS benefit. Alternative analysis approaches like the rank-preserving structural failure time (RPSFT) model (Robins, 1991; Ishak, 2014) have successfully been used to estimate the true treatment effect in similar trials designs with crossover.
Aims
Estimate the treatment effect in OS adjusted for the impact of crossover
Methods
Three methods of OS analysis were compared (data cut-off date 01July2014): 1) ITT analysis calculating the OS according to initial randomization including data after crossover (as pre-specified), 2) Censoring subjects at the time point of crossover, and 3) RPSFT.
Methods 1 and 2 commonly underestimate the true OS benefit. The RPSFT model estimates the counterfactual event time of the control arm that would have been observed without crossover and provides the estimate of treatment effect adjusted for the impact of crossover.
Results
110 pts were randomized to each arm. 38% (n=42) of pts on PBO + R crossed over to receive single-agent IDELA following PD according to the initial study design. An additional 40% (n=44) of subjects on PBO + R crossed over to receive idelalisib in the absence of prior PD according to an amended protocol after the study was stopped due to overwhelming PFS efficacy. The median follow-up for the presented analyses was 12.5 months (mos) for pts initially on IDELA + R and 11.1 mos for PBO + R. There were 17 deaths in the IDELA + R group, and 40 deaths in the PBO + R group. Analysis results from each method are presented in the table below:
Summary
IDELA+R demonstrated significant and consistent improvement in OS regardless of the analysis method. The median OS and HR as estimated by the RPSFT model reduced the bias associated with the idelalisib treatment effect compared to the ITT analysis or the censoring approach.
Keyword(s): Chronic lymphocytic leukemia, Clinical outcome, Phase III, PI3K

Session topic: CLL: Refining outcomes
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:45 to 14.06.2015 09:00
Location: Room A7
Background
A Phase 3 trial evaluated idelalisib (IDELA) + rituximab (R) vs placebo (PBO) + R in patients (pts) with relapsed CLL (Furman, NEJM, 2014). Using an extension study design, pts on the control arm were allowed to receive IDELA after progression (PD). Estimating the true overall survival (OS) benefit of the active arm of randomized studies with such a crossover design represents a challenging statistical problem. Intent-to-treat (ITT) analyses considering data from pts after crossover as well as the approach of censoring subjects at the time point of crossover may significantly underestimate the OS benefit. Alternative analysis approaches like the rank-preserving structural failure time (RPSFT) model (Robins, 1991; Ishak, 2014) have successfully been used to estimate the true treatment effect in similar trials designs with crossover.
Aims
Estimate the treatment effect in OS adjusted for the impact of crossover
Methods
Three methods of OS analysis were compared (data cut-off date 01July2014): 1) ITT analysis calculating the OS according to initial randomization including data after crossover (as pre-specified), 2) Censoring subjects at the time point of crossover, and 3) RPSFT.
Methods 1 and 2 commonly underestimate the true OS benefit. The RPSFT model estimates the counterfactual event time of the control arm that would have been observed without crossover and provides the estimate of treatment effect adjusted for the impact of crossover.
Results
110 pts were randomized to each arm. 38% (n=42) of pts on PBO + R crossed over to receive single-agent IDELA following PD according to the initial study design. An additional 40% (n=44) of subjects on PBO + R crossed over to receive idelalisib in the absence of prior PD according to an amended protocol after the study was stopped due to overwhelming PFS efficacy. The median follow-up for the presented analyses was 12.5 months (mos) for pts initially on IDELA + R and 11.1 mos for PBO + R. There were 17 deaths in the IDELA + R group, and 40 deaths in the PBO + R group. Analysis results from each method are presented in the table below:
Summary
IDELA+R demonstrated significant and consistent improvement in OS regardless of the analysis method. The median OS and HR as estimated by the RPSFT model reduced the bias associated with the idelalisib treatment effect compared to the ITT analysis or the censoring approach.
Keyword(s): Chronic lymphocytic leukemia, Clinical outcome, Phase III, PI3K

Session topic: CLL: Refining outcomes
{{ help_message }}
{{filter}}


