Department of Oncology and Children’s Research Centre

Contributions
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 09:00 to 14.06.2015 09:15
Location: Room Stolz 1
Background
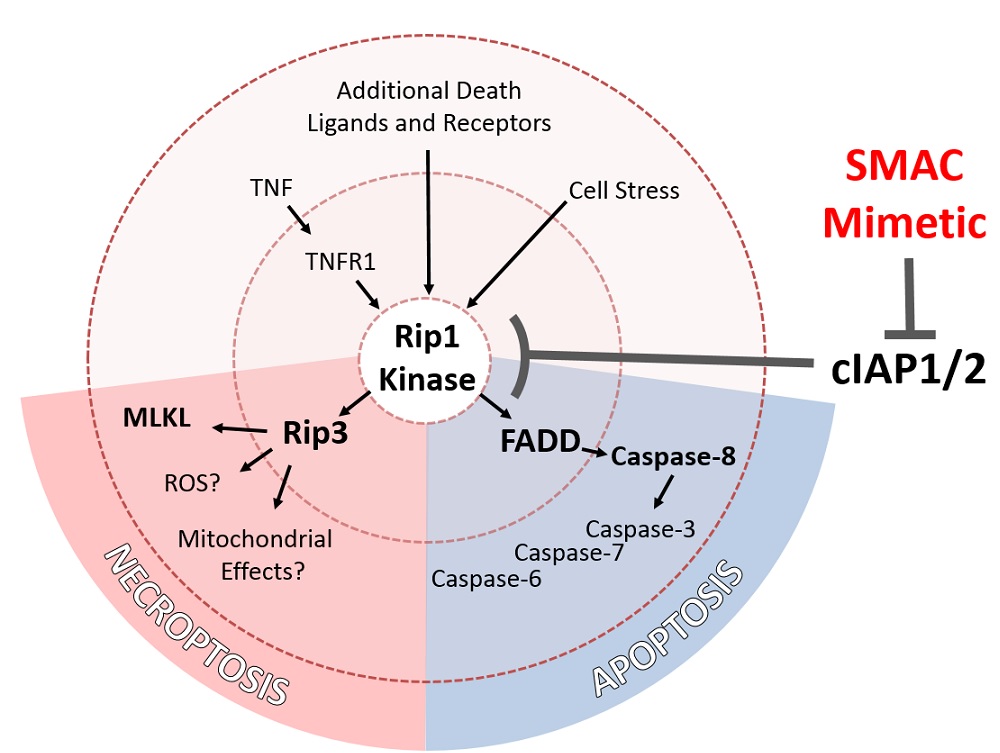
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, and although current drug treatment is effective in most cases there are few effective second-line drugs available for drug-resistant disease. SMAC mimetics (SMs) are an emerging class of novel chemotherapeutics, which activate cell death by inhibiting key anti-apoptotic cellular inhibitors of apoptosis proteins (cIAP1, cIAP2, and xIAP).
Aims
To elucidate the potency and mechanisms of cell death induction by SMAC mimetics in pediatric ALL.
Methods
Using an in vitro screening approach, we assessed the sensitivity of 51 patient derived ALL xenografts to a preclinical SM compound (Birinapant). We applied specific inhibitors of apoptotic and necroptotic cell death as well as a CRISPR based genetic approach to map the mechanisms of cell death induction by SM treatment in patient derived ALL samples as well as ALL cell lines. Finally, we examined the ability of SM to selectively kill ALL cells in an in vivo xenograft model of refractory ALL.
Results
We show that a significant proportion of patient-derived ALL samples are highly sensitive to SM-induced death. SM sensitivity does not correlate with patient risk grouping and includes both relapsed and refractory samples. Pharmacological inhibition of distinct cell death pathways indicates that SM treatment induces both apoptotic and necroptotic cell death. While a minority of ALL samples showed either apoptotic or necroptotic features, a majority of cases had simultaneous induction of both cell death phenotypes. Examination of distinct cell death parameters such as DNA-fragmentation and loss of plasma membrane integrity by FACS and microscopy suggests that both apoptosis and necroptosis occur simultaneously within a given cell population after SM treatment. Lenti-CRISPR mediated gene disruption in ALL cell lines demonstrates that irrespective of the cell death phenotype, induction of death requires Rip1-kinase in all cases. Rip1 in turn activates Rip3-dependent necroptosis and/or Caspase-8-dependent apoptosis. Blockade of both death pathways through gene disruption or inhibitor treatment was required to restore cell viability after SM treatment. Strong anti-leukemic activity of SM was confirmed in vivo in a xenograft mouse model of refractory ALL. Treatment of transplanted mice with SM not only delayed leukemia progression, but also completely eliminated leukemia burden in established disease for several weeks following treatment.
Summary
This data shows that apoptotic and necroptotic cell death pathways are not mutually exclusive phenomena, but can be activated simultaneously in chemotherapy-resistant ALL. Because SM can induce both pathways of cell death in parallel, these agents have a strong potential for anti-leukemic therapy in refractory childhood ALL.
Keyword(s): Acute lymphoblastic leukemia, Apoptosis
Session topic: Towards targeted therapy in ALL
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 09:00 to 14.06.2015 09:15
Location: Room Stolz 1
Background
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, and although current drug treatment is effective in most cases there are few effective second-line drugs available for drug-resistant disease. SMAC mimetics (SMs) are an emerging class of novel chemotherapeutics, which activate cell death by inhibiting key anti-apoptotic cellular inhibitors of apoptosis proteins (cIAP1, cIAP2, and xIAP).
Aims
To elucidate the potency and mechanisms of cell death induction by SMAC mimetics in pediatric ALL.
Methods
Using an in vitro screening approach, we assessed the sensitivity of 51 patient derived ALL xenografts to a preclinical SM compound (Birinapant). We applied specific inhibitors of apoptotic and necroptotic cell death as well as a CRISPR based genetic approach to map the mechanisms of cell death induction by SM treatment in patient derived ALL samples as well as ALL cell lines. Finally, we examined the ability of SM to selectively kill ALL cells in an in vivo xenograft model of refractory ALL.
Results
We show that a significant proportion of patient-derived ALL samples are highly sensitive to SM-induced death. SM sensitivity does not correlate with patient risk grouping and includes both relapsed and refractory samples. Pharmacological inhibition of distinct cell death pathways indicates that SM treatment induces both apoptotic and necroptotic cell death. While a minority of ALL samples showed either apoptotic or necroptotic features, a majority of cases had simultaneous induction of both cell death phenotypes. Examination of distinct cell death parameters such as DNA-fragmentation and loss of plasma membrane integrity by FACS and microscopy suggests that both apoptosis and necroptosis occur simultaneously within a given cell population after SM treatment. Lenti-CRISPR mediated gene disruption in ALL cell lines demonstrates that irrespective of the cell death phenotype, induction of death requires Rip1-kinase in all cases. Rip1 in turn activates Rip3-dependent necroptosis and/or Caspase-8-dependent apoptosis. Blockade of both death pathways through gene disruption or inhibitor treatment was required to restore cell viability after SM treatment. Strong anti-leukemic activity of SM was confirmed in vivo in a xenograft mouse model of refractory ALL. Treatment of transplanted mice with SM not only delayed leukemia progression, but also completely eliminated leukemia burden in established disease for several weeks following treatment.
Summary
This data shows that apoptotic and necroptotic cell death pathways are not mutually exclusive phenomena, but can be activated simultaneously in chemotherapy-resistant ALL. Because SM can induce both pathways of cell death in parallel, these agents have a strong potential for anti-leukemic therapy in refractory childhood ALL.
Keyword(s): Acute lymphoblastic leukemia, Apoptosis

Session topic: Towards targeted therapy in ALL


