Leukemia

Contributions
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:00 to 13.06.2015 12:15
Location: Room A8
Background
AZA is a DNA methyltransferase (DNMT) inhibitor with a modest response rate (20-25%) and duration (4 months) in MF. Ruxolitinib and azacytidine may target distinct clinical and pathological manifestations of myelofibrosis.
Aims
To determine the efficacy and safety of the combination in pts with MF.
Methods
A sequential approach with single-agent RUX 15 mg orally twice daily (if platelets 100-200) or 20 mg twice daily (if platelets >200) continuously in 28-day cycles for the first 3 months followed by the addition of AZA 25 mg/m2 on days 1-5 of each 28-day cycle starting cycle 4 was adopted. The AZA dosage could be gradually increased to a maximum of 75 mg/m2. Pts would be treated on study for 15 months followed by continuation of the combination off-study at the discretion of the treating physician.
Results
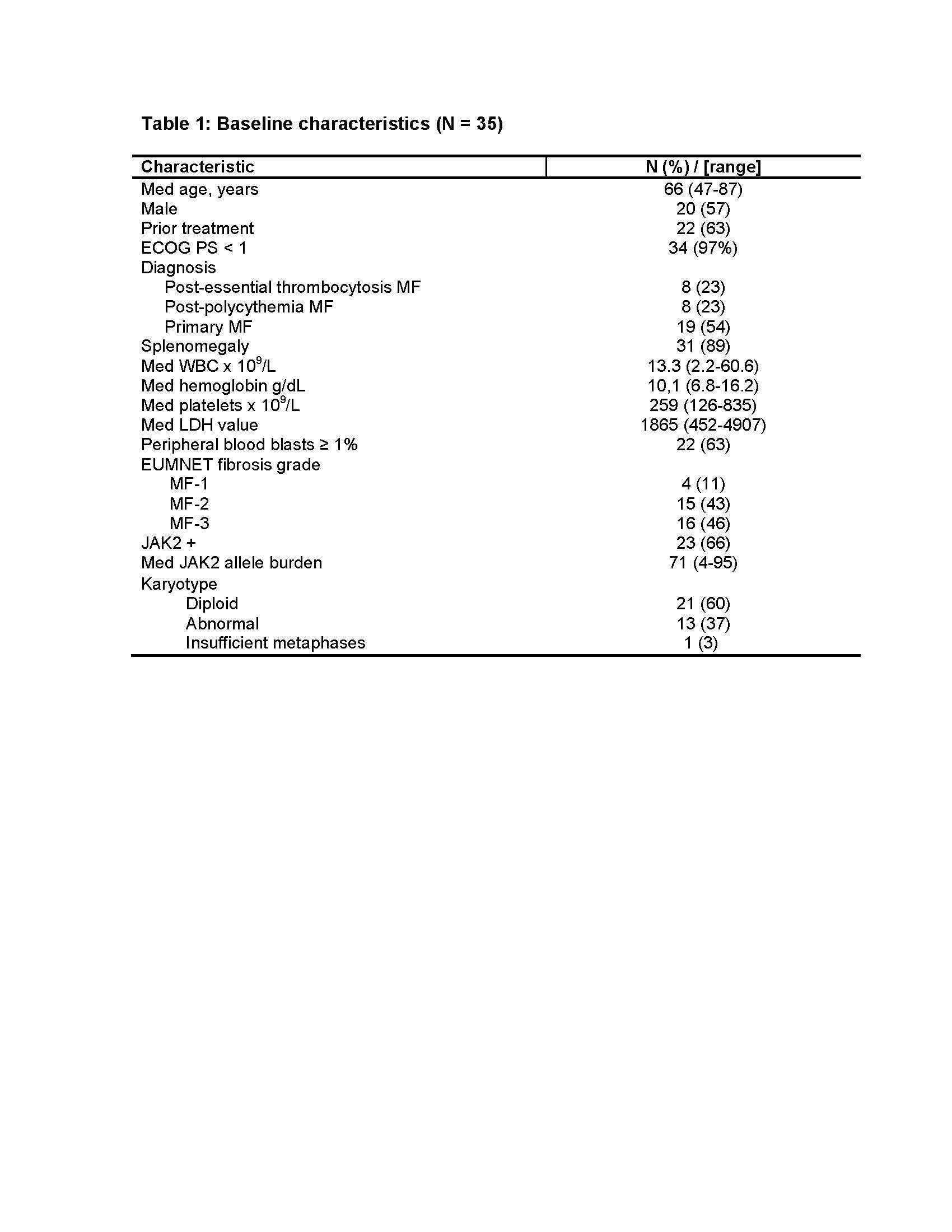
35 pts were enrolled between March 2013 and October 2014. 22 (63%) had received a median of 2 (1-3) prior therapies for MF. 31 pts remain alive after a med follow-up of 15.4 (3.4-22.8) months. Study is ongoing.
28 pts have been on the study for at least 6 months and are evaluable for response. International Working Group for Myelofibrosis Research and Treatment 2013 (IWG-MRT) objective responses were noted in 23 (82%), including PR in 1, CI for spleen and total symptom score (TSS) in 5 (18), CI for TSS and hemoglobin in 2 (7), CI for TSS only in 9 (32), and CI for spleen only in 6 (21). Responses occurred in 14 of 18 (78%) previously treated patients and 9 of 10 (90%) untreated pts (P=0.42). Median time to all responses was 1.0 month (0.8-7.5 months). Median time to CI in spleen size was 1.9 months (0.9-6.6), to CI TSS was 1.0 months (0.8-7.5), and CI Hb was 2.1 months (1.1-8.9). A reduction in the baseline JAK2V617F allele burden was noted in 11 of 13 serially evaluable responders with 2 of the responders demonstrating a >50% reduction in allele burden. Serial evaluation of bone marrow fibrosis revealed a documented reduction in EUMNET fibrosis score in 6 of 22 (27%) evaluable responders, after a median of 8 months (5-13) on therapy.
Only 5 of the 35 (14%) pts have not required a dose interruption/dose adjustment. The med time to first dose interruption/dose adjustment was 28 days (6-634). Among the 30 pts who needed a dose interruption/adjustment, 27 needed it within 3 months of initiation of the combination. The first change was a dose reduction in 17 pts and a dose interruption in 5 pts. AZA was the agent interrupted in 4 of these pts: 1 never started AZA, 1 didn’t resume AZA and 2 resumed AZA at same dose. The reasons for the first dose interruption were thrombocytopenia (n=2), neutropenia (n=1), knee replacement (n=1), and pneumonia (n=1). The first change was a dose increase in 8 pts. AZA was the first drug to be increased in 1 and RUX in 7. The reasons for dose increase were leukocytosis (n=3), progressive splenomegaly (n=1), thrombocytosis (n=2), and suboptimal response (n=3).
At the time of submission, 8 patients completed the predefined 15 months on the study, and 20 pts remain on study. Reasons for discontinuation in the remaining 7 pts included AML transformation (n=2), toxicity (n=2), lack of response (n=1), death (n=1), and patient preference (n=1). 4 pts experienced grade 3/4 non-hematological toxicity including fatigue (n=2), nausea (n=1), pneumonia (n=1), respectively.
Summary
Administration of RUX with AZA was feasible and resulted in improved response rate. The sequential addition of AZA was well tolerated with a lower incidence of early discontinuation than what has been seen with other ruxolitinib combination therapies. A sequential rather than concomitant approach may be considered when administering ruxolitinib combinations.
Keyword(s): Myelofibrosis
Session topic: MPN: Prognosis and treatment
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:00 to 13.06.2015 12:15
Location: Room A8
Background
AZA is a DNA methyltransferase (DNMT) inhibitor with a modest response rate (20-25%) and duration (4 months) in MF. Ruxolitinib and azacytidine may target distinct clinical and pathological manifestations of myelofibrosis.
Aims
To determine the efficacy and safety of the combination in pts with MF.
Methods
A sequential approach with single-agent RUX 15 mg orally twice daily (if platelets 100-200) or 20 mg twice daily (if platelets >200) continuously in 28-day cycles for the first 3 months followed by the addition of AZA 25 mg/m2 on days 1-5 of each 28-day cycle starting cycle 4 was adopted. The AZA dosage could be gradually increased to a maximum of 75 mg/m2. Pts would be treated on study for 15 months followed by continuation of the combination off-study at the discretion of the treating physician.
Results
35 pts were enrolled between March 2013 and October 2014. 22 (63%) had received a median of 2 (1-3) prior therapies for MF. 31 pts remain alive after a med follow-up of 15.4 (3.4-22.8) months. Study is ongoing.
28 pts have been on the study for at least 6 months and are evaluable for response. International Working Group for Myelofibrosis Research and Treatment 2013 (IWG-MRT) objective responses were noted in 23 (82%), including PR in 1, CI for spleen and total symptom score (TSS) in 5 (18), CI for TSS and hemoglobin in 2 (7), CI for TSS only in 9 (32), and CI for spleen only in 6 (21). Responses occurred in 14 of 18 (78%) previously treated patients and 9 of 10 (90%) untreated pts (P=0.42). Median time to all responses was 1.0 month (0.8-7.5 months). Median time to CI in spleen size was 1.9 months (0.9-6.6), to CI TSS was 1.0 months (0.8-7.5), and CI Hb was 2.1 months (1.1-8.9). A reduction in the baseline JAK2V617F allele burden was noted in 11 of 13 serially evaluable responders with 2 of the responders demonstrating a >50% reduction in allele burden. Serial evaluation of bone marrow fibrosis revealed a documented reduction in EUMNET fibrosis score in 6 of 22 (27%) evaluable responders, after a median of 8 months (5-13) on therapy.
Only 5 of the 35 (14%) pts have not required a dose interruption/dose adjustment. The med time to first dose interruption/dose adjustment was 28 days (6-634). Among the 30 pts who needed a dose interruption/adjustment, 27 needed it within 3 months of initiation of the combination. The first change was a dose reduction in 17 pts and a dose interruption in 5 pts. AZA was the agent interrupted in 4 of these pts: 1 never started AZA, 1 didn’t resume AZA and 2 resumed AZA at same dose. The reasons for the first dose interruption were thrombocytopenia (n=2), neutropenia (n=1), knee replacement (n=1), and pneumonia (n=1). The first change was a dose increase in 8 pts. AZA was the first drug to be increased in 1 and RUX in 7. The reasons for dose increase were leukocytosis (n=3), progressive splenomegaly (n=1), thrombocytosis (n=2), and suboptimal response (n=3).
At the time of submission, 8 patients completed the predefined 15 months on the study, and 20 pts remain on study. Reasons for discontinuation in the remaining 7 pts included AML transformation (n=2), toxicity (n=2), lack of response (n=1), death (n=1), and patient preference (n=1). 4 pts experienced grade 3/4 non-hematological toxicity including fatigue (n=2), nausea (n=1), pneumonia (n=1), respectively.
Summary
Administration of RUX with AZA was feasible and resulted in improved response rate. The sequential addition of AZA was well tolerated with a lower incidence of early discontinuation than what has been seen with other ruxolitinib combination therapies. A sequential rather than concomitant approach may be considered when administering ruxolitinib combinations.
Keyword(s): Myelofibrosis

Session topic: MPN: Prognosis and treatment


