Department of Haematology

Contributions
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:30 to 13.06.2015 11:45
Location: Room A8
Background
The observational EXELS study (NCT00567502) is the largest prospective cohort of high-risk patients (pts) with essential thrombocythemia (ET) reported to date.
Aims
Study objectives included safety, pregnancy outcomes, and efficacy (measured by incidence of thrombohemorrhagic events and platelet reduction) of anagrelide (ANA) compared with other cytoreductive therapies (CRTs). The multivariate (MV) analysis aimed to identify risk factors for thrombohemorrhagic/transformation events.
Methods
Eligible pts were enrolled across 13 European countries between 2005 and 2009. Data were collected every 6 mo for 5 yrs. Event rates are presented as number of pts per 100 pt?yrs exposure and by treatment at registration. A Cox proportional hazards model was used for the MV analysis to predict events based on certain risk factors. All pts provided informed consent. This study was sponsored by Shire Pharmaceutical Development Ltd.
Results
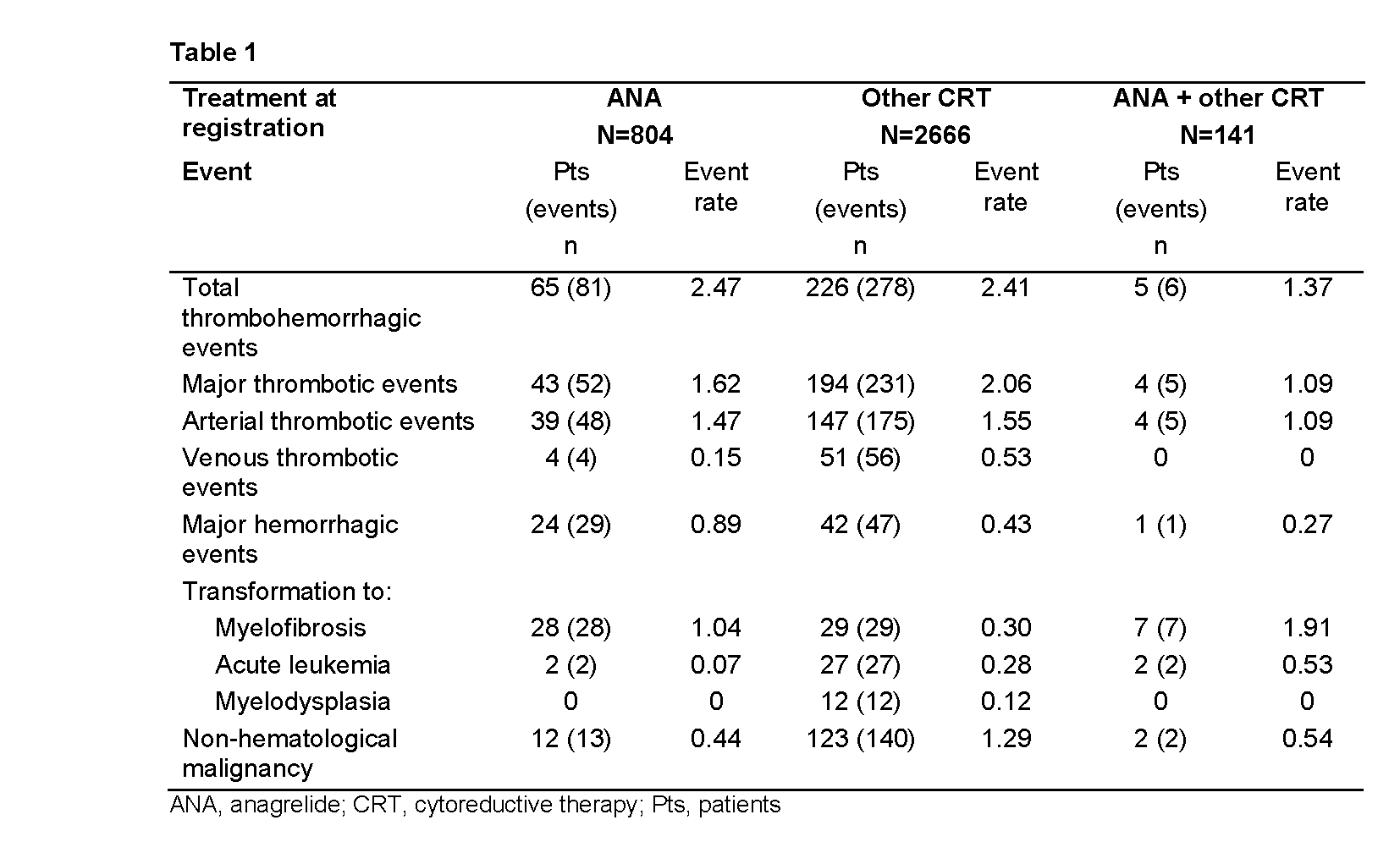
3649 pts were categorized according to treatment at registration: ANA (n=804), ANA + other CRT (n=141), other CRT (n=2666), and no CRT (n=38). More than 80% of pts received either hydroxycarbamide (HC) or ANA. Median age was lower in the ANA (55.5 yrs) vs other CRT group (70.0 yrs). Thrombohemorrhagic and malignancy event rates are displayed in Table 1. Median platelet counts in the ANA vs other CRT group were 443 vs 428x109/L, respectively, at baseline, and 402 vs 430x109/L, respectively, at the time of major thrombotic event. Median white blood cell (WBC) counts in the ANA vs other CRT group were 8.8 vs 6.0x109/L, respectively, at baseline, and 9.0 vs 6.2x109/L, respectively, at the time of major thrombotic event. 64 pts transformed to myelofibrosis (MF) and 31 to acute leukemia (AL). In pts who had only ever received either ANA or HC, the rate of transformation to MF was higher in the ANA vs HC group (0.78 vs 0.17), whereas transformation to AL was higher in the HC vs ANA group (0.22 vs 0). Non?hematological malignancy event rate was higher in the other CRT vs ANA group.
MV analysis identified both a history of thrombohemorrhagic events and age ≥65 yrs at baseline as risk factors for predicting thrombohemorrhagic (hazard ratio [HR] 1.91 and 1.67), major thrombotic (HR 2.05 and 2.14), arterial thrombotic (HR 1.81 and 1.90), venous thrombotic (HR 3.70 and 3.27), and transformation to AL/myelodysplasia (MDS) events (HR 2.17 and 3.36). Presence of baseline cardiovascular risk factors increased the risk of major thrombotic (HR 1.38) and arterial thrombotic events (HR 1.65), while baseline hypertension was a risk factor for thrombohemorrhagic events (HR 1.33). ET diagnostic criteria (WHO vs PVSG) and aspirin at registration were not identified as risk factors for thrombohemorrhagic events. Time since diagnosis (5–<10 yrs; ≥10 yrs) and baseline platelet count increase of 100 units above normal (≤450x109/L) were both identified as risk factors for transformation to MF (HR 3.38; 4.38 and 5.34).
Summary
In this large prospective study population, MV analysis identified the following baseline risk factors for thrombohemorrhagic events: history of thrombohemorrhagic events, age ≥65 yrs, cardiovascular risk factors, and hypertension. In contrast, within each treatment group, the baseline platelet and WBC counts do not appear to be significantly different at the time of major thrombotic events. A history of thrombohemorrhagic events, age ≥65 yrs, time since diagnosis, and platelet count increase of 100 units above normal were identified as baseline risk factors for transformation to AL/MDS/MF.
Keyword(s): Essential Thrombocytemia, Risk factor, Transformation
Session topic: MPN: Prognosis and treatment
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:30 to 13.06.2015 11:45
Location: Room A8
Background
The observational EXELS study (NCT00567502) is the largest prospective cohort of high-risk patients (pts) with essential thrombocythemia (ET) reported to date.
Aims
Study objectives included safety, pregnancy outcomes, and efficacy (measured by incidence of thrombohemorrhagic events and platelet reduction) of anagrelide (ANA) compared with other cytoreductive therapies (CRTs). The multivariate (MV) analysis aimed to identify risk factors for thrombohemorrhagic/transformation events.
Methods
Eligible pts were enrolled across 13 European countries between 2005 and 2009. Data were collected every 6 mo for 5 yrs. Event rates are presented as number of pts per 100 pt?yrs exposure and by treatment at registration. A Cox proportional hazards model was used for the MV analysis to predict events based on certain risk factors. All pts provided informed consent. This study was sponsored by Shire Pharmaceutical Development Ltd.
Results
3649 pts were categorized according to treatment at registration: ANA (n=804), ANA + other CRT (n=141), other CRT (n=2666), and no CRT (n=38). More than 80% of pts received either hydroxycarbamide (HC) or ANA. Median age was lower in the ANA (55.5 yrs) vs other CRT group (70.0 yrs). Thrombohemorrhagic and malignancy event rates are displayed in Table 1. Median platelet counts in the ANA vs other CRT group were 443 vs 428x109/L, respectively, at baseline, and 402 vs 430x109/L, respectively, at the time of major thrombotic event. Median white blood cell (WBC) counts in the ANA vs other CRT group were 8.8 vs 6.0x109/L, respectively, at baseline, and 9.0 vs 6.2x109/L, respectively, at the time of major thrombotic event. 64 pts transformed to myelofibrosis (MF) and 31 to acute leukemia (AL). In pts who had only ever received either ANA or HC, the rate of transformation to MF was higher in the ANA vs HC group (0.78 vs 0.17), whereas transformation to AL was higher in the HC vs ANA group (0.22 vs 0). Non?hematological malignancy event rate was higher in the other CRT vs ANA group.
MV analysis identified both a history of thrombohemorrhagic events and age ≥65 yrs at baseline as risk factors for predicting thrombohemorrhagic (hazard ratio [HR] 1.91 and 1.67), major thrombotic (HR 2.05 and 2.14), arterial thrombotic (HR 1.81 and 1.90), venous thrombotic (HR 3.70 and 3.27), and transformation to AL/myelodysplasia (MDS) events (HR 2.17 and 3.36). Presence of baseline cardiovascular risk factors increased the risk of major thrombotic (HR 1.38) and arterial thrombotic events (HR 1.65), while baseline hypertension was a risk factor for thrombohemorrhagic events (HR 1.33). ET diagnostic criteria (WHO vs PVSG) and aspirin at registration were not identified as risk factors for thrombohemorrhagic events. Time since diagnosis (5–<10 yrs; ≥10 yrs) and baseline platelet count increase of 100 units above normal (≤450x109/L) were both identified as risk factors for transformation to MF (HR 3.38; 4.38 and 5.34).
Summary
In this large prospective study population, MV analysis identified the following baseline risk factors for thrombohemorrhagic events: history of thrombohemorrhagic events, age ≥65 yrs, cardiovascular risk factors, and hypertension. In contrast, within each treatment group, the baseline platelet and WBC counts do not appear to be significantly different at the time of major thrombotic events. A history of thrombohemorrhagic events, age ≥65 yrs, time since diagnosis, and platelet count increase of 100 units above normal were identified as baseline risk factors for transformation to AL/MDS/MF.
Keyword(s): Essential Thrombocytemia, Risk factor, Transformation

Session topic: MPN: Prognosis and treatment


