Cellular Biotechnologies and Haematology

Contributions
Type: Oral Presentation + travel grant
Presentation during EHA20: From 12.06.2015 12:30 to 12.06.2015 12:45
Location: Room A8
Background
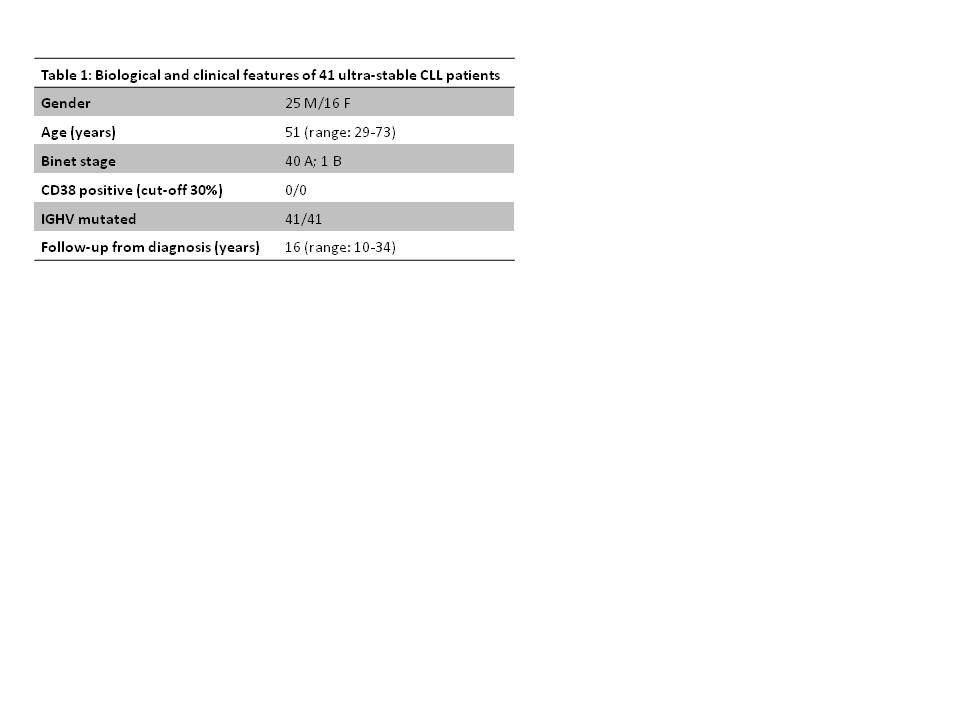
Chronic lymphocytic leukemia (CLL) shows an extremely heterogeneous clinical course. Beside cases with aggressive disease requiring immediate treatment and those with an initial indolent phase followed by progression, there are patients who do not progress for decades. Next generation sequencing (NGS) technologies have allowed a further understanding of the molecular complexity of CLL. In the present study, we characterized 41 ultra-stable CLL cases by whole exome sequencing (WES), ultra-deep NGS and copy number aberration (CNA) analysis. Ultra-stable disease was defined as absence of progression for at least 10 years from diagnosis.
Aims
To investigate the so far unexplored mutational profile and CNA load of ultra-stable CLL patients.
Methods
Peripheral blood samples from 20 ultra-stable CLL patients were used for WES analysis (Illumina HiSeq2000), including paired germline DNA in 14. Sanger sequencing (ABI PRISM 3100) was used to validate WES mutations and to screen the recurrency of mutated genes identified in ≥2 cases by WES, in a second cohort of 21 ultra-stable CLL samples. Bad prognosticator genes (NOTCH1-BIRC3-SF3B1-TP53) were also investigated. Subclonal TP53 mutations were examined by ultra-deep NGS (Roche-454 GS Junior) in 36 cases. CNA analysis (Affymetrix Cytoscan HD arrays) was performed in 30 cases.
Results
WES analysis of the 14 cases having paired germline DNA predicted 83 non-silent somatic mutations in 81 genes, with a mutation load of 6 mutations/case (range: 1-12). The remaining 6 cases without germline DNA were analyzed to assess the recurrence of mutations identified in the former cohort and to investigate those with known significance. Three genes were recurrently mutated: RBM46, KLHL6, UBR5. Since RBM46 was the most recurrent among ultra-stable CLL (3 cases, 15%) and never reported in other CLL WES studies, Sanger sequencing of the whole codifying region was performed on the screening cohort with no additional mutated case identified. Interestingly, none of the genes with known adverse prognostic impact was found mutated in 41 ultra-stable CLL, including ATM and MYD88 in the WES cohort. Unexpectedly, ultra-deep-NGS of TP53 revealed subclonal mutations in 2/36 cases (5.5%). One case after 19 years from diagnosis showed 4 mutations with a median allele frequency (AF) of 1.55% (range 0.9-1.79), corrected for tumor representation, and developed a clinical progression soon after the inclusion in the study; after therapy, a clonal TP53 mutation expansion occurred. The second case showed 1 subclonal mutation (AF 5.2%) after 29 years from diagnosis; for the subsequent 4 years she remained in clinical spontaneous regression of CLL but developed a breast cancer. AS-PCR validation of these subclonal mutations is ongoing. CNA analysis identified 31 lesions represented by 90% of losses and 10% of gains, giving a CNA load of 1/case. Twelve cases (40%) showed no lesion, 9 (30%) showed isolated del(13q), 5 cases (17%) del(13q) with additional non-canonical CNAs and 4 cases (13%) a median of 1 non-canonical CNAs. There was no recurrent CNA beside 13q deletion, as well as no CNA with known poor prognostic significance.
Summary
Ultra-stable CLL show no driver mutations or CNAs. No new recurrent lesion associated to a highly stable course was identified. We found two cases with TP53 subclonal mutations with an extremely divergent clinical history, which could be due to the subclonal architecture complexity and to the chemotherapy selective pressure in the case with clonal evolution.
Keyword(s): Chronic lymphocytic leukemia, Genomics
Session topic: CLL - Biology: Interacting determinants of CLL ontogeny and evolution
Type: Oral Presentation + travel grant
Presentation during EHA20: From 12.06.2015 12:30 to 12.06.2015 12:45
Location: Room A8
Background
Chronic lymphocytic leukemia (CLL) shows an extremely heterogeneous clinical course. Beside cases with aggressive disease requiring immediate treatment and those with an initial indolent phase followed by progression, there are patients who do not progress for decades. Next generation sequencing (NGS) technologies have allowed a further understanding of the molecular complexity of CLL. In the present study, we characterized 41 ultra-stable CLL cases by whole exome sequencing (WES), ultra-deep NGS and copy number aberration (CNA) analysis. Ultra-stable disease was defined as absence of progression for at least 10 years from diagnosis.
Aims
To investigate the so far unexplored mutational profile and CNA load of ultra-stable CLL patients.
Methods
Peripheral blood samples from 20 ultra-stable CLL patients were used for WES analysis (Illumina HiSeq2000), including paired germline DNA in 14. Sanger sequencing (ABI PRISM 3100) was used to validate WES mutations and to screen the recurrency of mutated genes identified in ≥2 cases by WES, in a second cohort of 21 ultra-stable CLL samples. Bad prognosticator genes (NOTCH1-BIRC3-SF3B1-TP53) were also investigated. Subclonal TP53 mutations were examined by ultra-deep NGS (Roche-454 GS Junior) in 36 cases. CNA analysis (Affymetrix Cytoscan HD arrays) was performed in 30 cases.
Results
WES analysis of the 14 cases having paired germline DNA predicted 83 non-silent somatic mutations in 81 genes, with a mutation load of 6 mutations/case (range: 1-12). The remaining 6 cases without germline DNA were analyzed to assess the recurrence of mutations identified in the former cohort and to investigate those with known significance. Three genes were recurrently mutated: RBM46, KLHL6, UBR5. Since RBM46 was the most recurrent among ultra-stable CLL (3 cases, 15%) and never reported in other CLL WES studies, Sanger sequencing of the whole codifying region was performed on the screening cohort with no additional mutated case identified. Interestingly, none of the genes with known adverse prognostic impact was found mutated in 41 ultra-stable CLL, including ATM and MYD88 in the WES cohort. Unexpectedly, ultra-deep-NGS of TP53 revealed subclonal mutations in 2/36 cases (5.5%). One case after 19 years from diagnosis showed 4 mutations with a median allele frequency (AF) of 1.55% (range 0.9-1.79), corrected for tumor representation, and developed a clinical progression soon after the inclusion in the study; after therapy, a clonal TP53 mutation expansion occurred. The second case showed 1 subclonal mutation (AF 5.2%) after 29 years from diagnosis; for the subsequent 4 years she remained in clinical spontaneous regression of CLL but developed a breast cancer. AS-PCR validation of these subclonal mutations is ongoing. CNA analysis identified 31 lesions represented by 90% of losses and 10% of gains, giving a CNA load of 1/case. Twelve cases (40%) showed no lesion, 9 (30%) showed isolated del(13q), 5 cases (17%) del(13q) with additional non-canonical CNAs and 4 cases (13%) a median of 1 non-canonical CNAs. There was no recurrent CNA beside 13q deletion, as well as no CNA with known poor prognostic significance.
Summary
Ultra-stable CLL show no driver mutations or CNAs. No new recurrent lesion associated to a highly stable course was identified. We found two cases with TP53 subclonal mutations with an extremely divergent clinical history, which could be due to the subclonal architecture complexity and to the chemotherapy selective pressure in the case with clonal evolution.
Keyword(s): Chronic lymphocytic leukemia, Genomics

Session topic: CLL - Biology: Interacting determinants of CLL ontogeny and evolution


