EFFECT OF CARFILZOMIB, LENALIDOMIDE, AND DEXAMETHASONE VS LENALIDOMIDE AND DEXAMETHASONE IN PATIENTS WITH RELAPSED MULTIPLE MYELOMA BY LINE OF THERAPY: INTERIM RESULTS FROM THE PHASE 3 ASPIRE STUDY
(Abstract release date: 05/21/15)
EHA Library. Dimopoulos M. 06/13/15; 103134; S427
Disclosure(s): National and Kapodistrian University of Athens
Prof. Dr. Meletios Dimopoulos
Contributions
Contributions
Abstract
Abstract: S427
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:45 to 13.06.2015 12:00
Location: Room A2+3
Background
Previously reported results from ASPIRE (NCT01080391; N=792 patients) showed that carfilzomib, lenalidomide, and dexamethasone (KRd) significantly improved progression-free survival (PFS) vs lenalidomide and dexamethasone (Rd) in patients with relapsed multiple myeloma, with a favorable benefit-risk profile (Stewart et al. N Engl J Med 2015;372:142–52).
Aims
A secondary analysis of efficacy and safety results from patients treated with KRd or Rd after first relapse (1 prior line of therapy) vs ≥2 prior lines of therapy in the ASPIRE study is presented.
Methods
Adults with relapsed multiple myeloma who received 1–3 prior lines were eligible. Patients were randomized (1:1) to KRd or Rd. All patients received lenalidomide (25 mg) on days 1–21 and dexamethasone (40 mg) on days 1, 8, 15, and 22 of a 28?day cycle. Patients in the KRd arm received carfilzomib as a 10?min infusion on days 1, 2, 8, 9, 15, and 16 during cycles 1–12 (20 mg/m2 [days 1 and 2 of cycle 1]; 27 mg/m2 thereafter). Carfilzomib was omitted on days 8 and 9 during cycles 13–18 and was not administered beyond 18 cycles. All patients provided informed consent.
Results
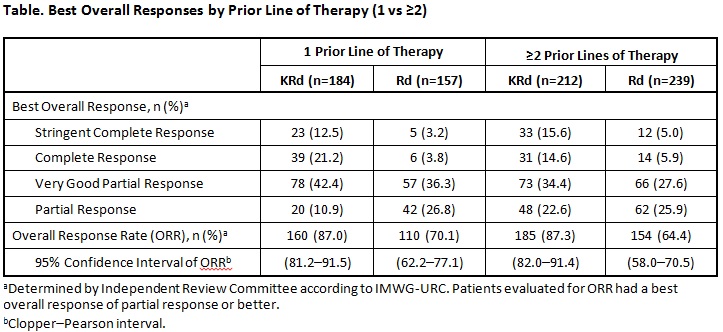
Median PFS for patients receiving 1 prior line (n=341) was 29.6 months (95% confidence interval [CI]: 23.2–33.5) for KRd vs 17.6 months (95% CI: 15.0–22.2) for Rd (hazard ratio [HR]: 0.694; P=.0083). Median PFS for patients receiving ≥2 prior lines (n=451) was 25.8 months (95% CI: 22.2–31.0) for KRd vs 16.7 months (95% CI: 13.9–22.0) for Rd (HR: 0.688; P=.0017). Best overall responses in patients who received 1 vs ≥2 prior lines of therapy are presented in the Table. Overall response rates (partial response or better) were 87.0% (KRd) vs 70.1% (Rd) in patients with 1 prior line and 87.3% (KRd) vs 64.4% (Rd) in patients with ≥2 prior lines. In patients with 1 prior line, 33.7% (KRd) vs 7.0% (Rd) achieved a complete response (CR) or better, including 12.5% (KRd) and 3.2% (Rd) who achieved a stringent complete response (sCR). In patients with ≥2 prior lines, 30.2% (KRd) vs 10.9% (Rd) achieved a CR or better, including 15.6% (KRd) and 5.0% (Rd) who achieved a sCR. Adverse events (AEs) grade ≥3 were reported in 85.7% (KRd) and 79.9% (Rd) of patients who received 1 prior line of therapy and 81.9% (KRd) and 81.3% (Rd) of patients who received ≥2 prior lines. AEs of interest (grade ≥3; grouped terms) included dyspnea (1 prior line: 2.7% [KRd] and 2.6% [Rd]; ≥2 prior lines: 3.3% [KRd] and 1.7% [Rd]); cardiac failure (1 prior line: 3.3% [KRd] and 1.9% [Rd]; ≥2 prior lines: 4.3% [KRd] and 1.7% [Rd]); ischemic heart disease (1 prior line: 4.9% [KRd] and 1.3% [Rd]; ≥2 prior lines: 1.9% [KRd] and 2.6% [Rd]); hypertension (preferred term; 1 prior line: 3.8% [KRd] and 0.6% [Rd]; ≥2 prior lines: 4.8% [KRd] and 2.6% [Rd]); and acute renal failure (1 prior line: 3.3% [KRd] and 3.2% [Rd]; ≥2 prior lines: 3.3% [KRd] and 3.0% [Rd]).
Summary
The use of KRd led to a 1-year improvement in median PFS vs Rd after first relapse, and a 9-month improvement in median PFS vs Rd in patients with ≥2 prior lines of therapy, with similar HRs. KRd had a favorable benefit–risk profile compared with Rd after 1 and ≥2 prior lines of therapy in patients with relapsed multiple myeloma.
Keyword(s): Immunomodulatory thalidomide analog, Myeloma, Phase III, Proteasome inhibitor
Session topic: Multiple myeloma: Clinical studies 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:45 to 13.06.2015 12:00
Location: Room A2+3
Background
Previously reported results from ASPIRE (NCT01080391; N=792 patients) showed that carfilzomib, lenalidomide, and dexamethasone (KRd) significantly improved progression-free survival (PFS) vs lenalidomide and dexamethasone (Rd) in patients with relapsed multiple myeloma, with a favorable benefit-risk profile (Stewart et al. N Engl J Med 2015;372:142–52).
Aims
A secondary analysis of efficacy and safety results from patients treated with KRd or Rd after first relapse (1 prior line of therapy) vs ≥2 prior lines of therapy in the ASPIRE study is presented.
Methods
Adults with relapsed multiple myeloma who received 1–3 prior lines were eligible. Patients were randomized (1:1) to KRd or Rd. All patients received lenalidomide (25 mg) on days 1–21 and dexamethasone (40 mg) on days 1, 8, 15, and 22 of a 28?day cycle. Patients in the KRd arm received carfilzomib as a 10?min infusion on days 1, 2, 8, 9, 15, and 16 during cycles 1–12 (20 mg/m2 [days 1 and 2 of cycle 1]; 27 mg/m2 thereafter). Carfilzomib was omitted on days 8 and 9 during cycles 13–18 and was not administered beyond 18 cycles. All patients provided informed consent.
Results
Median PFS for patients receiving 1 prior line (n=341) was 29.6 months (95% confidence interval [CI]: 23.2–33.5) for KRd vs 17.6 months (95% CI: 15.0–22.2) for Rd (hazard ratio [HR]: 0.694; P=.0083). Median PFS for patients receiving ≥2 prior lines (n=451) was 25.8 months (95% CI: 22.2–31.0) for KRd vs 16.7 months (95% CI: 13.9–22.0) for Rd (HR: 0.688; P=.0017). Best overall responses in patients who received 1 vs ≥2 prior lines of therapy are presented in the Table. Overall response rates (partial response or better) were 87.0% (KRd) vs 70.1% (Rd) in patients with 1 prior line and 87.3% (KRd) vs 64.4% (Rd) in patients with ≥2 prior lines. In patients with 1 prior line, 33.7% (KRd) vs 7.0% (Rd) achieved a complete response (CR) or better, including 12.5% (KRd) and 3.2% (Rd) who achieved a stringent complete response (sCR). In patients with ≥2 prior lines, 30.2% (KRd) vs 10.9% (Rd) achieved a CR or better, including 15.6% (KRd) and 5.0% (Rd) who achieved a sCR. Adverse events (AEs) grade ≥3 were reported in 85.7% (KRd) and 79.9% (Rd) of patients who received 1 prior line of therapy and 81.9% (KRd) and 81.3% (Rd) of patients who received ≥2 prior lines. AEs of interest (grade ≥3; grouped terms) included dyspnea (1 prior line: 2.7% [KRd] and 2.6% [Rd]; ≥2 prior lines: 3.3% [KRd] and 1.7% [Rd]); cardiac failure (1 prior line: 3.3% [KRd] and 1.9% [Rd]; ≥2 prior lines: 4.3% [KRd] and 1.7% [Rd]); ischemic heart disease (1 prior line: 4.9% [KRd] and 1.3% [Rd]; ≥2 prior lines: 1.9% [KRd] and 2.6% [Rd]); hypertension (preferred term; 1 prior line: 3.8% [KRd] and 0.6% [Rd]; ≥2 prior lines: 4.8% [KRd] and 2.6% [Rd]); and acute renal failure (1 prior line: 3.3% [KRd] and 3.2% [Rd]; ≥2 prior lines: 3.3% [KRd] and 3.0% [Rd]).
Summary
The use of KRd led to a 1-year improvement in median PFS vs Rd after first relapse, and a 9-month improvement in median PFS vs Rd in patients with ≥2 prior lines of therapy, with similar HRs. KRd had a favorable benefit–risk profile compared with Rd after 1 and ≥2 prior lines of therapy in patients with relapsed multiple myeloma.
Keyword(s): Immunomodulatory thalidomide analog, Myeloma, Phase III, Proteasome inhibitor

Session topic: Multiple myeloma: Clinical studies 2
Abstract: S427
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:45 to 13.06.2015 12:00
Location: Room A2+3
Background
Previously reported results from ASPIRE (NCT01080391; N=792 patients) showed that carfilzomib, lenalidomide, and dexamethasone (KRd) significantly improved progression-free survival (PFS) vs lenalidomide and dexamethasone (Rd) in patients with relapsed multiple myeloma, with a favorable benefit-risk profile (Stewart et al. N Engl J Med 2015;372:142–52).
Aims
A secondary analysis of efficacy and safety results from patients treated with KRd or Rd after first relapse (1 prior line of therapy) vs ≥2 prior lines of therapy in the ASPIRE study is presented.
Methods
Adults with relapsed multiple myeloma who received 1–3 prior lines were eligible. Patients were randomized (1:1) to KRd or Rd. All patients received lenalidomide (25 mg) on days 1–21 and dexamethasone (40 mg) on days 1, 8, 15, and 22 of a 28?day cycle. Patients in the KRd arm received carfilzomib as a 10?min infusion on days 1, 2, 8, 9, 15, and 16 during cycles 1–12 (20 mg/m2 [days 1 and 2 of cycle 1]; 27 mg/m2 thereafter). Carfilzomib was omitted on days 8 and 9 during cycles 13–18 and was not administered beyond 18 cycles. All patients provided informed consent.
Results
Median PFS for patients receiving 1 prior line (n=341) was 29.6 months (95% confidence interval [CI]: 23.2–33.5) for KRd vs 17.6 months (95% CI: 15.0–22.2) for Rd (hazard ratio [HR]: 0.694; P=.0083). Median PFS for patients receiving ≥2 prior lines (n=451) was 25.8 months (95% CI: 22.2–31.0) for KRd vs 16.7 months (95% CI: 13.9–22.0) for Rd (HR: 0.688; P=.0017). Best overall responses in patients who received 1 vs ≥2 prior lines of therapy are presented in the Table. Overall response rates (partial response or better) were 87.0% (KRd) vs 70.1% (Rd) in patients with 1 prior line and 87.3% (KRd) vs 64.4% (Rd) in patients with ≥2 prior lines. In patients with 1 prior line, 33.7% (KRd) vs 7.0% (Rd) achieved a complete response (CR) or better, including 12.5% (KRd) and 3.2% (Rd) who achieved a stringent complete response (sCR). In patients with ≥2 prior lines, 30.2% (KRd) vs 10.9% (Rd) achieved a CR or better, including 15.6% (KRd) and 5.0% (Rd) who achieved a sCR. Adverse events (AEs) grade ≥3 were reported in 85.7% (KRd) and 79.9% (Rd) of patients who received 1 prior line of therapy and 81.9% (KRd) and 81.3% (Rd) of patients who received ≥2 prior lines. AEs of interest (grade ≥3; grouped terms) included dyspnea (1 prior line: 2.7% [KRd] and 2.6% [Rd]; ≥2 prior lines: 3.3% [KRd] and 1.7% [Rd]); cardiac failure (1 prior line: 3.3% [KRd] and 1.9% [Rd]; ≥2 prior lines: 4.3% [KRd] and 1.7% [Rd]); ischemic heart disease (1 prior line: 4.9% [KRd] and 1.3% [Rd]; ≥2 prior lines: 1.9% [KRd] and 2.6% [Rd]); hypertension (preferred term; 1 prior line: 3.8% [KRd] and 0.6% [Rd]; ≥2 prior lines: 4.8% [KRd] and 2.6% [Rd]); and acute renal failure (1 prior line: 3.3% [KRd] and 3.2% [Rd]; ≥2 prior lines: 3.3% [KRd] and 3.0% [Rd]).
Summary
The use of KRd led to a 1-year improvement in median PFS vs Rd after first relapse, and a 9-month improvement in median PFS vs Rd in patients with ≥2 prior lines of therapy, with similar HRs. KRd had a favorable benefit–risk profile compared with Rd after 1 and ≥2 prior lines of therapy in patients with relapsed multiple myeloma.
Keyword(s): Immunomodulatory thalidomide analog, Myeloma, Phase III, Proteasome inhibitor

Session topic: Multiple myeloma: Clinical studies 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:45 to 13.06.2015 12:00
Location: Room A2+3
Background
Previously reported results from ASPIRE (NCT01080391; N=792 patients) showed that carfilzomib, lenalidomide, and dexamethasone (KRd) significantly improved progression-free survival (PFS) vs lenalidomide and dexamethasone (Rd) in patients with relapsed multiple myeloma, with a favorable benefit-risk profile (Stewart et al. N Engl J Med 2015;372:142–52).
Aims
A secondary analysis of efficacy and safety results from patients treated with KRd or Rd after first relapse (1 prior line of therapy) vs ≥2 prior lines of therapy in the ASPIRE study is presented.
Methods
Adults with relapsed multiple myeloma who received 1–3 prior lines were eligible. Patients were randomized (1:1) to KRd or Rd. All patients received lenalidomide (25 mg) on days 1–21 and dexamethasone (40 mg) on days 1, 8, 15, and 22 of a 28?day cycle. Patients in the KRd arm received carfilzomib as a 10?min infusion on days 1, 2, 8, 9, 15, and 16 during cycles 1–12 (20 mg/m2 [days 1 and 2 of cycle 1]; 27 mg/m2 thereafter). Carfilzomib was omitted on days 8 and 9 during cycles 13–18 and was not administered beyond 18 cycles. All patients provided informed consent.
Results
Median PFS for patients receiving 1 prior line (n=341) was 29.6 months (95% confidence interval [CI]: 23.2–33.5) for KRd vs 17.6 months (95% CI: 15.0–22.2) for Rd (hazard ratio [HR]: 0.694; P=.0083). Median PFS for patients receiving ≥2 prior lines (n=451) was 25.8 months (95% CI: 22.2–31.0) for KRd vs 16.7 months (95% CI: 13.9–22.0) for Rd (HR: 0.688; P=.0017). Best overall responses in patients who received 1 vs ≥2 prior lines of therapy are presented in the Table. Overall response rates (partial response or better) were 87.0% (KRd) vs 70.1% (Rd) in patients with 1 prior line and 87.3% (KRd) vs 64.4% (Rd) in patients with ≥2 prior lines. In patients with 1 prior line, 33.7% (KRd) vs 7.0% (Rd) achieved a complete response (CR) or better, including 12.5% (KRd) and 3.2% (Rd) who achieved a stringent complete response (sCR). In patients with ≥2 prior lines, 30.2% (KRd) vs 10.9% (Rd) achieved a CR or better, including 15.6% (KRd) and 5.0% (Rd) who achieved a sCR. Adverse events (AEs) grade ≥3 were reported in 85.7% (KRd) and 79.9% (Rd) of patients who received 1 prior line of therapy and 81.9% (KRd) and 81.3% (Rd) of patients who received ≥2 prior lines. AEs of interest (grade ≥3; grouped terms) included dyspnea (1 prior line: 2.7% [KRd] and 2.6% [Rd]; ≥2 prior lines: 3.3% [KRd] and 1.7% [Rd]); cardiac failure (1 prior line: 3.3% [KRd] and 1.9% [Rd]; ≥2 prior lines: 4.3% [KRd] and 1.7% [Rd]); ischemic heart disease (1 prior line: 4.9% [KRd] and 1.3% [Rd]; ≥2 prior lines: 1.9% [KRd] and 2.6% [Rd]); hypertension (preferred term; 1 prior line: 3.8% [KRd] and 0.6% [Rd]; ≥2 prior lines: 4.8% [KRd] and 2.6% [Rd]); and acute renal failure (1 prior line: 3.3% [KRd] and 3.2% [Rd]; ≥2 prior lines: 3.3% [KRd] and 3.0% [Rd]).
Summary
The use of KRd led to a 1-year improvement in median PFS vs Rd after first relapse, and a 9-month improvement in median PFS vs Rd in patients with ≥2 prior lines of therapy, with similar HRs. KRd had a favorable benefit–risk profile compared with Rd after 1 and ≥2 prior lines of therapy in patients with relapsed multiple myeloma.
Keyword(s): Immunomodulatory thalidomide analog, Myeloma, Phase III, Proteasome inhibitor

Session topic: Multiple myeloma: Clinical studies 2
{{ help_message }}
{{filter}}


