
Contributions
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:45 to 14.06.2015 09:00
Location: Room Strauss 1
Background
CML treatment has been significantly improved by the tyrosine kinase inhibitor (TKI) imatinib, but at least one-third of patients will eventually fail imatinib treatment2, 3 and a significant proportion of these will progress towards blast crisis (BC), which is usually rapidly fatal. Suppression of PP2A activity by the inhibitors SET and CIP2A is important in the pathogenesis and progression of chronic myeloid leukaemia (CML). We have previously shown that CML patients with a high diagnostic CIP2A protein level who then receive imatinib have a high risk of progressing to blast crisis.
Aims
The aim of this study was to investigate if high CIP2A also confers a high progression rate in patients receiving a second generation (2G) TKI dasatinib or nilotinib from diagnosis.
Methods
The diagnostic CIP2A protein level was assessed by flow cytometry in 74 newly diagnosed chronic phase patients, who were subsequently treated with either imatinib or a 2G TKI. ROC analysis was used to define a CIP2A cut off level to stratify cases into high and low CIP2A groups. All have been seen since original diagnosis of chronic phase CML at our centre and have been followed for at least 9 months (median follow-up: 50 months).
Results
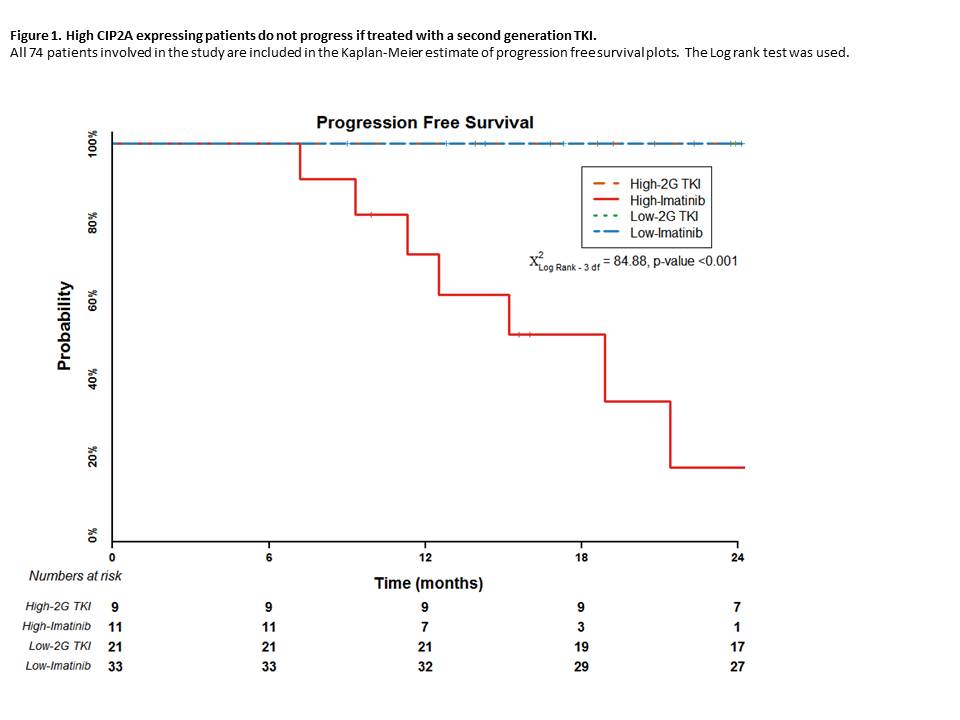
For patients with high diagnostic CIP2A level treated with imatinib, the overall and progression-free survival probability at 24 months was 41% and 17% respectively, compared to 100% for patients in any other group (p<0.001, Figure 1). Disease progression to blast crisis only occurred in those patients with a high diagnostic level of CIP2A and treated with imatinib. All progressions occurred within 32 months from diagnosis with the median time to progression being 12.5 months and disease progression was not associated with the development of BCR-ABL kinase domain mutations.
The cumulative complete cytogenetic response (CCR) rate for low CIP2A level imatinib treated patients was 85% at 18 months. In contrast, only a single patient with a high diagnostic CIP2A level achieved CCR (cumulative CCR rate of 9% at 18 months; p<0.001); this patient subsequently progressed. This deleterious effect of high CIP2A was not seen if patients were treated with a 2G TKI from diagnosis, where the estimated cumulative CCR rate at 18 months was 86% and 56% for the low and high CIP2A 2G TKI patients respectively. The cumulative rate of MR4 (BCR-ABL1/ABL transcript ratio of ≤0.01%) for low CIP2A imatinib treated patients was 16% at 18 months. No patient with a high diagnostic CIP2A level and treated with imatinib achieved MR4 (p=0.001). High CIP2A patients treated with a 2G TKI have a lower rate of MR4 compared to low CIP2A 2G TKI treated patients, 11% and 48% respectively. High diagnostic CIP2A levels in patients treated with a 2G TKI predicts for poor molecular response. Early molecular response (EMR, BCR-ABL1/ABL ratio of <10% at 3 months) is an excellent predictor of clinical outcome in imatinib treated patients. 55% of imatinib treated low CIP2A patients achieved an EMR, compared to only 9% of high CIP2A patients (p=0.01). 90% of low CIP2A patients treated with a 2G TKI achieved an EMR compared to 44% of those with a high CIP2A level.
Summary
In summary, patients with high diagnostic CIP2A levels and treated with a 2G TKI at initial diagnosis do not progress to BC, suggesting that if a patient has a high CIP2A level at diagnosis then they should not be treated with imatinib due to the high risk of disease progression. A high diagnostic level of CIP2A in patients treated with a 2G TKI can predict an inferior deep molecular response. There is therefore now a case for routine CIP2A testing at diagnosis. Further study to determine the true incidence of high CIP2A patients in the general CML population is needed.
Keyword(s): Blast crisis, Imatinib, Tyrosine kinase inhibitor
Session topic: CML: Molecular-cytogenetic diagnostics
Type: Oral Presentation
Presentation during EHA20: From 14.06.2015 08:45 to 14.06.2015 09:00
Location: Room Strauss 1
Background
CML treatment has been significantly improved by the tyrosine kinase inhibitor (TKI) imatinib, but at least one-third of patients will eventually fail imatinib treatment2, 3 and a significant proportion of these will progress towards blast crisis (BC), which is usually rapidly fatal. Suppression of PP2A activity by the inhibitors SET and CIP2A is important in the pathogenesis and progression of chronic myeloid leukaemia (CML). We have previously shown that CML patients with a high diagnostic CIP2A protein level who then receive imatinib have a high risk of progressing to blast crisis.
Aims
The aim of this study was to investigate if high CIP2A also confers a high progression rate in patients receiving a second generation (2G) TKI dasatinib or nilotinib from diagnosis.
Methods
The diagnostic CIP2A protein level was assessed by flow cytometry in 74 newly diagnosed chronic phase patients, who were subsequently treated with either imatinib or a 2G TKI. ROC analysis was used to define a CIP2A cut off level to stratify cases into high and low CIP2A groups. All have been seen since original diagnosis of chronic phase CML at our centre and have been followed for at least 9 months (median follow-up: 50 months).
Results
For patients with high diagnostic CIP2A level treated with imatinib, the overall and progression-free survival probability at 24 months was 41% and 17% respectively, compared to 100% for patients in any other group (p<0.001, Figure 1). Disease progression to blast crisis only occurred in those patients with a high diagnostic level of CIP2A and treated with imatinib. All progressions occurred within 32 months from diagnosis with the median time to progression being 12.5 months and disease progression was not associated with the development of BCR-ABL kinase domain mutations.
The cumulative complete cytogenetic response (CCR) rate for low CIP2A level imatinib treated patients was 85% at 18 months. In contrast, only a single patient with a high diagnostic CIP2A level achieved CCR (cumulative CCR rate of 9% at 18 months; p<0.001); this patient subsequently progressed. This deleterious effect of high CIP2A was not seen if patients were treated with a 2G TKI from diagnosis, where the estimated cumulative CCR rate at 18 months was 86% and 56% for the low and high CIP2A 2G TKI patients respectively. The cumulative rate of MR4 (BCR-ABL1/ABL transcript ratio of ≤0.01%) for low CIP2A imatinib treated patients was 16% at 18 months. No patient with a high diagnostic CIP2A level and treated with imatinib achieved MR4 (p=0.001). High CIP2A patients treated with a 2G TKI have a lower rate of MR4 compared to low CIP2A 2G TKI treated patients, 11% and 48% respectively. High diagnostic CIP2A levels in patients treated with a 2G TKI predicts for poor molecular response. Early molecular response (EMR, BCR-ABL1/ABL ratio of <10% at 3 months) is an excellent predictor of clinical outcome in imatinib treated patients. 55% of imatinib treated low CIP2A patients achieved an EMR, compared to only 9% of high CIP2A patients (p=0.01). 90% of low CIP2A patients treated with a 2G TKI achieved an EMR compared to 44% of those with a high CIP2A level.
Summary
In summary, patients with high diagnostic CIP2A levels and treated with a 2G TKI at initial diagnosis do not progress to BC, suggesting that if a patient has a high CIP2A level at diagnosis then they should not be treated with imatinib due to the high risk of disease progression. A high diagnostic level of CIP2A in patients treated with a 2G TKI can predict an inferior deep molecular response. There is therefore now a case for routine CIP2A testing at diagnosis. Further study to determine the true incidence of high CIP2A patients in the general CML population is needed.
Keyword(s): Blast crisis, Imatinib, Tyrosine kinase inhibitor

Session topic: CML: Molecular-cytogenetic diagnostics


