
Contributions
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:15 to 13.06.2015 12:30
Location: Room C2
Background
The Best conditioning regimen, i.e. one that is both safe and efficacious for treatment of patients with myelofibrosis (MF) is not known. After observing a higher relapse rate in the initial cohort receiving a reduced intensity regimen, we increased the intensity of the conditioning regimen for subsequent patients, hypothesizing that increased dose intensity delivered with pharmacokinetic- (PK-) dose guidance will reduce the relapse rate without increasing non-relapse mortality (NRM), and thereby improving overall outcome.
Aims
To report final results of a prospective phase II clinical trial of Fludarabine (Flu) and busulfan (bu) conditioning in MF patients with a median follow-up of 5 years.
Methods
Patients with advanced MF were eligible if they had adequate organ function and at least 9/10 HLA matched related or unrelated donor. All 46 patients received fludarabine 40 mg/m2 x 4 (day -5 to -2), and in the first 15 (bu low group) IV busulfan 130 mg/m2/day x 2 was given after the Flu dose on days -3,and -2). Of the remaining 31 (bu high group), 27 received IV busulfan dose to a target daily systemic exposure, AUC, of 4000 μmol-min x 4, given on day -5 to -2, (total course AUC of 16,000 μmol-min) and 4 patients received a fixed dose of 100 mg/m2/day x 4 (days -5 to -2), each Bu dose was preceded by the day’s Flu dose.
Results
23 males and 23 females with a median age of 58 years (27-74) had intermediate (27 Int-2, 1 Int-1) or high-risk (18) disease as per Dynamic international prognostic scoring system plus (DIPSS plus) criteria. Donors were matched sibs (19), matched unrelated (23), or mismatched unrelated (4).All patients engrafted with a median time to neutrophil engraftment of 13 (0-27) days and a median time to platelet engraftment of 24 (0-268) days. The Cumulative incidence (CI) of grade II-IV, grade III, and IV acute GVHD, and Chronic GVHD were 22%, 7%, and 40%, respectively.
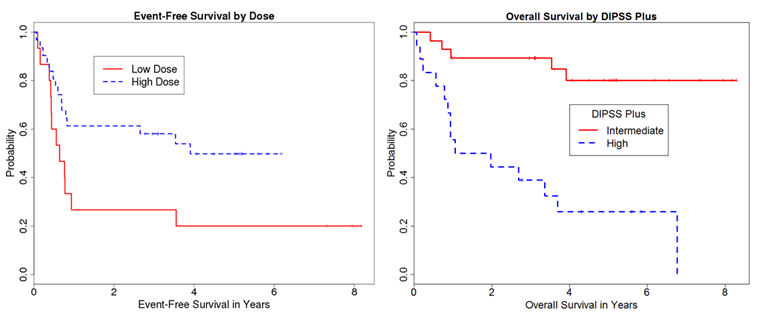
With a median follow up of surviving patients of 5.1 years (range 1-8.3 years), 3 year overall survival (OS), event-free survival (EFS), cumulative incidence (CI) of non-relapse mortality (NRM), and CI of relapse were 69%, 48%, 13%, and 39%, respectively. Multivariate Cox regression analysis showed that Bu-high dose (HR 0.44; p=0.07) was associated with lower relapse rate. Bu-high dose (HR 0.5; p=0.09), DIPSS plus high (HR 2.69; P=0.02) and Age (HR 1.05; P=0.08) were predictors of EFS (fig 1). DIPSS plus high (HR 5.99; P=0.001) and Age (HR 1.07; P=0.03) were adverse predictors of OS.
Summary
Allogeneic transplantation results in long-term survival in patients with myelofibrosis with better outcome seen in earlier phase of the disease. PK guided myeloablative busulfan (AUC 16,000 μmol.min) appears promising in reducing relapse rate without increasing non-relapse mortality.
Keyword(s): Allogeneic bone marrow transplant, Myelodysplasia, Myelofibrosis, Reduced intensity
Session topic: Stem cell transplantation: Clinical 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:15 to 13.06.2015 12:30
Location: Room C2
Background
The Best conditioning regimen, i.e. one that is both safe and efficacious for treatment of patients with myelofibrosis (MF) is not known. After observing a higher relapse rate in the initial cohort receiving a reduced intensity regimen, we increased the intensity of the conditioning regimen for subsequent patients, hypothesizing that increased dose intensity delivered with pharmacokinetic- (PK-) dose guidance will reduce the relapse rate without increasing non-relapse mortality (NRM), and thereby improving overall outcome.
Aims
To report final results of a prospective phase II clinical trial of Fludarabine (Flu) and busulfan (bu) conditioning in MF patients with a median follow-up of 5 years.
Methods
Patients with advanced MF were eligible if they had adequate organ function and at least 9/10 HLA matched related or unrelated donor. All 46 patients received fludarabine 40 mg/m2 x 4 (day -5 to -2), and in the first 15 (bu low group) IV busulfan 130 mg/m2/day x 2 was given after the Flu dose on days -3,and -2). Of the remaining 31 (bu high group), 27 received IV busulfan dose to a target daily systemic exposure, AUC, of 4000 μmol-min x 4, given on day -5 to -2, (total course AUC of 16,000 μmol-min) and 4 patients received a fixed dose of 100 mg/m2/day x 4 (days -5 to -2), each Bu dose was preceded by the day’s Flu dose.
Results
23 males and 23 females with a median age of 58 years (27-74) had intermediate (27 Int-2, 1 Int-1) or high-risk (18) disease as per Dynamic international prognostic scoring system plus (DIPSS plus) criteria. Donors were matched sibs (19), matched unrelated (23), or mismatched unrelated (4).All patients engrafted with a median time to neutrophil engraftment of 13 (0-27) days and a median time to platelet engraftment of 24 (0-268) days. The Cumulative incidence (CI) of grade II-IV, grade III, and IV acute GVHD, and Chronic GVHD were 22%, 7%, and 40%, respectively.
With a median follow up of surviving patients of 5.1 years (range 1-8.3 years), 3 year overall survival (OS), event-free survival (EFS), cumulative incidence (CI) of non-relapse mortality (NRM), and CI of relapse were 69%, 48%, 13%, and 39%, respectively. Multivariate Cox regression analysis showed that Bu-high dose (HR 0.44; p=0.07) was associated with lower relapse rate. Bu-high dose (HR 0.5; p=0.09), DIPSS plus high (HR 2.69; P=0.02) and Age (HR 1.05; P=0.08) were predictors of EFS (fig 1). DIPSS plus high (HR 5.99; P=0.001) and Age (HR 1.07; P=0.03) were adverse predictors of OS.
Summary
Allogeneic transplantation results in long-term survival in patients with myelofibrosis with better outcome seen in earlier phase of the disease. PK guided myeloablative busulfan (AUC 16,000 μmol.min) appears promising in reducing relapse rate without increasing non-relapse mortality.
Keyword(s): Allogeneic bone marrow transplant, Myelodysplasia, Myelofibrosis, Reduced intensity

Session topic: Stem cell transplantation: Clinical 2


