OPTIMAL DURATION OF ANTICOAGULANT THERAPY FOR THE TREATMENT OF CANCER-ASSOCIATED THROMBOSIS
(Abstract release date: 05/21/15)
EHA Library. Chai-adisaksopha C. 06/12/15; 103094; S142
Disclosure(s): McMaster UniversityMedicine

Chatree Chai-adisaksopha
Contributions
Contributions
Abstract
Abstract: S142
Type: Oral Presentation + travel grant
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room Stolz 1
Background
Cancer-associated thrombosis is associated with a high risk of recurrent thrombosis despite “usual intensity warfarin”; anticoagulant therapy, specifically low-molecular weight heparin, reduces the risk of recurrent thrombosis compared with warfarin. However, the optimal duration of treatment in patients with cancer associated thrombosis remains unclear.
Aims
To evaluate the effects of treatment duration on the outcomes in patients with cancer-associated thrombosis.
Methods
Consecutive patients with symptomatic venous thromboembolism [VTE] (deep vein thrombosis or pulmonary embolism) were prospectively enrolled in the RIETE registry. The patient were categorized into 2 group according to received treatment duration (< 6 months vs ≥ 6 months). All the patients were followed up to 5 years from treatment start date. The primary outcome was recurrent VTE and the secondary outcomes were all-cause mortality, VTE-related death and major bleeding.
Results
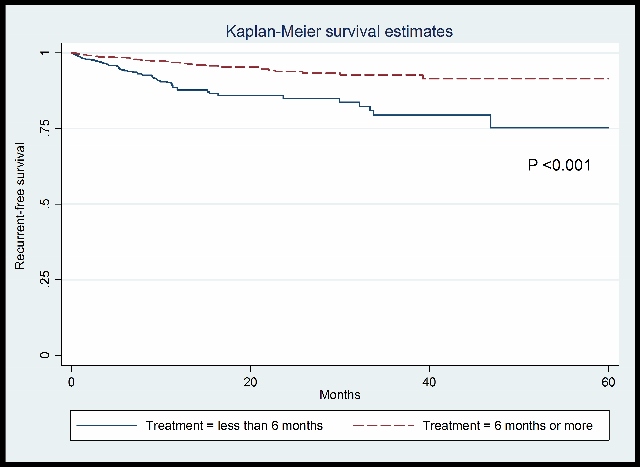
A total of 4,460 patients with cancer-associated thrombosis were enrolled in the analysis. Of these, 2,937 patients received anticoagulant for less than 6 months, and 1,523 received anticoagulants for more than 6 months. Patients receiving anticoagulant therapy for less than 6 months had an increased risk of recurrent thrombosis (hazard ratio [HR] 2.86, 95% confidence interval [CI] 2.22 to 3.70) when compared to patients received anticoagulant for more than 6 months. The risk of all-cause mortality and VTE-related death were significantly increased higher in patients treated for less than 6 months, HR 5.88 (95%CI 5.00 to 6.67) and HR 6.25 (95% CI 3.03 to 14.29), respectively. We did not observe increased major bleeding in patients who were treated for more than 6 months, HR 0.26 (95% CI 0.19 to 0.38).
Summary
We demonstrate that patients with cancer-associated thrombosis patients have a higher risk of recurrent VTE, VTE-related death and all-cause mortality if their anticoagulants are not continued beyond six months from diagnosis.
Keyword(s): Anticoagulants, Deep venous thrombosis, Pulmonary embolism, Thromboembolism
Session topic: Thrombosis and vascular biology
Type: Oral Presentation + travel grant
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room Stolz 1
Background
Cancer-associated thrombosis is associated with a high risk of recurrent thrombosis despite “usual intensity warfarin”; anticoagulant therapy, specifically low-molecular weight heparin, reduces the risk of recurrent thrombosis compared with warfarin. However, the optimal duration of treatment in patients with cancer associated thrombosis remains unclear.
Aims
To evaluate the effects of treatment duration on the outcomes in patients with cancer-associated thrombosis.
Methods
Consecutive patients with symptomatic venous thromboembolism [VTE] (deep vein thrombosis or pulmonary embolism) were prospectively enrolled in the RIETE registry. The patient were categorized into 2 group according to received treatment duration (< 6 months vs ≥ 6 months). All the patients were followed up to 5 years from treatment start date. The primary outcome was recurrent VTE and the secondary outcomes were all-cause mortality, VTE-related death and major bleeding.
Results
A total of 4,460 patients with cancer-associated thrombosis were enrolled in the analysis. Of these, 2,937 patients received anticoagulant for less than 6 months, and 1,523 received anticoagulants for more than 6 months. Patients receiving anticoagulant therapy for less than 6 months had an increased risk of recurrent thrombosis (hazard ratio [HR] 2.86, 95% confidence interval [CI] 2.22 to 3.70) when compared to patients received anticoagulant for more than 6 months. The risk of all-cause mortality and VTE-related death were significantly increased higher in patients treated for less than 6 months, HR 5.88 (95%CI 5.00 to 6.67) and HR 6.25 (95% CI 3.03 to 14.29), respectively. We did not observe increased major bleeding in patients who were treated for more than 6 months, HR 0.26 (95% CI 0.19 to 0.38).
Summary
We demonstrate that patients with cancer-associated thrombosis patients have a higher risk of recurrent VTE, VTE-related death and all-cause mortality if their anticoagulants are not continued beyond six months from diagnosis.
Keyword(s): Anticoagulants, Deep venous thrombosis, Pulmonary embolism, Thromboembolism

Session topic: Thrombosis and vascular biology
Abstract: S142
Type: Oral Presentation + travel grant
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room Stolz 1
Background
Cancer-associated thrombosis is associated with a high risk of recurrent thrombosis despite “usual intensity warfarin”; anticoagulant therapy, specifically low-molecular weight heparin, reduces the risk of recurrent thrombosis compared with warfarin. However, the optimal duration of treatment in patients with cancer associated thrombosis remains unclear.
Aims
To evaluate the effects of treatment duration on the outcomes in patients with cancer-associated thrombosis.
Methods
Consecutive patients with symptomatic venous thromboembolism [VTE] (deep vein thrombosis or pulmonary embolism) were prospectively enrolled in the RIETE registry. The patient were categorized into 2 group according to received treatment duration (< 6 months vs ≥ 6 months). All the patients were followed up to 5 years from treatment start date. The primary outcome was recurrent VTE and the secondary outcomes were all-cause mortality, VTE-related death and major bleeding.
Results
A total of 4,460 patients with cancer-associated thrombosis were enrolled in the analysis. Of these, 2,937 patients received anticoagulant for less than 6 months, and 1,523 received anticoagulants for more than 6 months. Patients receiving anticoagulant therapy for less than 6 months had an increased risk of recurrent thrombosis (hazard ratio [HR] 2.86, 95% confidence interval [CI] 2.22 to 3.70) when compared to patients received anticoagulant for more than 6 months. The risk of all-cause mortality and VTE-related death were significantly increased higher in patients treated for less than 6 months, HR 5.88 (95%CI 5.00 to 6.67) and HR 6.25 (95% CI 3.03 to 14.29), respectively. We did not observe increased major bleeding in patients who were treated for more than 6 months, HR 0.26 (95% CI 0.19 to 0.38).
Summary
We demonstrate that patients with cancer-associated thrombosis patients have a higher risk of recurrent VTE, VTE-related death and all-cause mortality if their anticoagulants are not continued beyond six months from diagnosis.
Keyword(s): Anticoagulants, Deep venous thrombosis, Pulmonary embolism, Thromboembolism

Session topic: Thrombosis and vascular biology
Type: Oral Presentation + travel grant
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room Stolz 1
Background
Cancer-associated thrombosis is associated with a high risk of recurrent thrombosis despite “usual intensity warfarin”; anticoagulant therapy, specifically low-molecular weight heparin, reduces the risk of recurrent thrombosis compared with warfarin. However, the optimal duration of treatment in patients with cancer associated thrombosis remains unclear.
Aims
To evaluate the effects of treatment duration on the outcomes in patients with cancer-associated thrombosis.
Methods
Consecutive patients with symptomatic venous thromboembolism [VTE] (deep vein thrombosis or pulmonary embolism) were prospectively enrolled in the RIETE registry. The patient were categorized into 2 group according to received treatment duration (< 6 months vs ≥ 6 months). All the patients were followed up to 5 years from treatment start date. The primary outcome was recurrent VTE and the secondary outcomes were all-cause mortality, VTE-related death and major bleeding.
Results
A total of 4,460 patients with cancer-associated thrombosis were enrolled in the analysis. Of these, 2,937 patients received anticoagulant for less than 6 months, and 1,523 received anticoagulants for more than 6 months. Patients receiving anticoagulant therapy for less than 6 months had an increased risk of recurrent thrombosis (hazard ratio [HR] 2.86, 95% confidence interval [CI] 2.22 to 3.70) when compared to patients received anticoagulant for more than 6 months. The risk of all-cause mortality and VTE-related death were significantly increased higher in patients treated for less than 6 months, HR 5.88 (95%CI 5.00 to 6.67) and HR 6.25 (95% CI 3.03 to 14.29), respectively. We did not observe increased major bleeding in patients who were treated for more than 6 months, HR 0.26 (95% CI 0.19 to 0.38).
Summary
We demonstrate that patients with cancer-associated thrombosis patients have a higher risk of recurrent VTE, VTE-related death and all-cause mortality if their anticoagulants are not continued beyond six months from diagnosis.
Keyword(s): Anticoagulants, Deep venous thrombosis, Pulmonary embolism, Thromboembolism

Session topic: Thrombosis and vascular biology
{{ help_message }}
{{filter}}


