RESULTS FROM TWO PHASE 3 STUDIES OF POST-TRANSPLANT BORTEZOMIB (BTZ) CONSOLIDATION VS OBSERVATION (OBS) IN PATIENTS WITH NEWLY DIAGNOSED MULTIPLE MYELOMA (NDMM)
(Abstract release date: 05/21/15)
EHA Library. Einsele H. 06/13/15; 103092; S426
Disclosure(s): Julius Maximilians Universität WürzburgMedizinische Klinik und Poliklinik II

Prof. Hermann Einsele
Contributions
Contributions
Abstract
Abstract: S426
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:30 to 13.06.2015 11:45
Location: Room A2+3
Background
HDT followed by ASCT remains the gold standard for treatment of NDMM patients who are able to tolerate the procedure. Following ASCT, consolidation with novel agents for a fixed time period (or number of cycles) can improve outcomes. However, there are currently few published data on BTZ consolidation therapy, although a study by the Nordic Myeloma Study Group has reported results in BTZ-naïve patients (Mellqvist et al Blood 2013).
Aims
The results from two large randomized controlled phase 3 studies were combined to investigate BTZ consolidation or OBS in patients with NDMM both with and without prior BTZ treatment.
Methods
MMY3012 (NCT00416273; 222 patients aged ≤60 yrs) and MMY3013 (NCT00416208; 158 patients aged 61–75 yrs) recruited adults with NDMM who underwent induction therapy followed by ASCT. Patients were randomized 1:1 to receive BTZ consolidation (1.6 mg/m² IV days 1, 8, 15, 22; 4 x 35-day cycles) or OBS, 60–120 days after ASCT. The primary endpoint was progression-free survival (PFS) from the start of induction; secondary endpoints included response rate, overall survival (OS), and safety. Factors affecting PFS were also assessed by post-hoc multivariate analysis. Responses were assessed per EBMT response criteria, with VGPR as an additional category. Adverse events (AEs) were graded per NCI-CTCAE v3.0.
Results
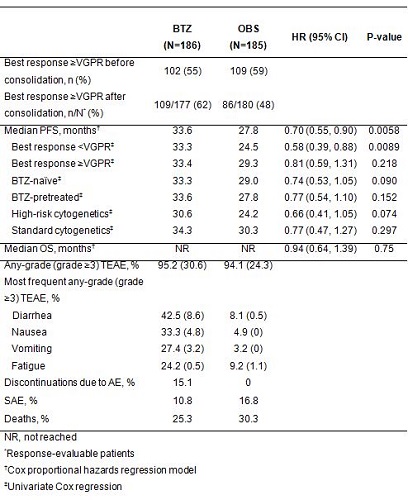
In 371 randomized patients, median age was 59 yrs (35–76); 62% were male, 14% / 84% were Durie-Salmon stage II / III. Of the 278 patients assessed for cytogenetics, 37% were classified as high-risk (32% del13q, 10% t(4;14), 6% del17p). Overall, 50% of patients had received prior BTZ therapy; the most common induction regimen was VCD (40%). Others included dexamethasone/idarubicin (14%), dexamethasone (13%), VAD (9%), adriamycin/dexamethasone (6%) and VD (6%). In the overall study population, patients who received BTZ-based induction showed a trend towards improved PFS (HR 1.24; 95% CI: 0.97, 1.59; p=0.084) by unadjusted Cox regression analysis. Outcomes for patients receiving BTZ consolidation or OBS only are shown in the table (median follow-up 50 months from start of induction). PFS was significantly improved by approximately 6 months, but there was no improvement in OS. In a post-hoc exploratory multivariate analysis, BTZ consolidation remained a predictor for PFS (HR 0.69; 95% CI: 0.51, 0.93; p=0.016). There appeared to be an increased risk of progression in patients with high-risk cytogenetics (HR 1.45; 95% CI: 1.05, 1.99; p=0.025) or those with60 had no effect on PFS (HR 1.24; 95% CI: 0.87, 1.77; p=0.240); neither did the use of non-BTZ induction (HR 1.17; 95% CI: 0.83, 1.66; p=0.375).
Summary
These data indicated that a fixed period (4 cycles) of BTZ consolidation was beneficial in NDMM patients with or without prior BTZ exposure. A higher proportion of patients achieved ≥VGPR after BTZ consolidation than OBS. PFS was significantly improved, but there was no improvement in OS at data cutoff, possibly related to limited follow-up and the use of effective salvage options. Subgroups that seemed to benefit from BTZ consolidation were patients with
Keyword(s): Bortezomib, Consolidation, Multiple myeloma, Phase III
Session topic: Multiple myeloma: Clinical studies 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:30 to 13.06.2015 11:45
Location: Room A2+3
Background
HDT followed by ASCT remains the gold standard for treatment of NDMM patients who are able to tolerate the procedure. Following ASCT, consolidation with novel agents for a fixed time period (or number of cycles) can improve outcomes. However, there are currently few published data on BTZ consolidation therapy, although a study by the Nordic Myeloma Study Group has reported results in BTZ-naïve patients (Mellqvist et al Blood 2013).
Aims
The results from two large randomized controlled phase 3 studies were combined to investigate BTZ consolidation or OBS in patients with NDMM both with and without prior BTZ treatment.
Methods
MMY3012 (NCT00416273; 222 patients aged ≤60 yrs) and MMY3013 (NCT00416208; 158 patients aged 61–75 yrs) recruited adults with NDMM who underwent induction therapy followed by ASCT. Patients were randomized 1:1 to receive BTZ consolidation (1.6 mg/m² IV days 1, 8, 15, 22; 4 x 35-day cycles) or OBS, 60–120 days after ASCT. The primary endpoint was progression-free survival (PFS) from the start of induction; secondary endpoints included response rate, overall survival (OS), and safety. Factors affecting PFS were also assessed by post-hoc multivariate analysis. Responses were assessed per EBMT response criteria, with VGPR as an additional category. Adverse events (AEs) were graded per NCI-CTCAE v3.0.
Results
In 371 randomized patients, median age was 59 yrs (35–76); 62% were male, 14% / 84% were Durie-Salmon stage II / III. Of the 278 patients assessed for cytogenetics, 37% were classified as high-risk (32% del13q, 10% t(4;14), 6% del17p). Overall, 50% of patients had received prior BTZ therapy; the most common induction regimen was VCD (40%). Others included dexamethasone/idarubicin (14%), dexamethasone (13%), VAD (9%), adriamycin/dexamethasone (6%) and VD (6%). In the overall study population, patients who received BTZ-based induction showed a trend towards improved PFS (HR 1.24; 95% CI: 0.97, 1.59; p=0.084) by unadjusted Cox regression analysis. Outcomes for patients receiving BTZ consolidation or OBS only are shown in the table (median follow-up 50 months from start of induction). PFS was significantly improved by approximately 6 months, but there was no improvement in OS. In a post-hoc exploratory multivariate analysis, BTZ consolidation remained a predictor for PFS (HR 0.69; 95% CI: 0.51, 0.93; p=0.016). There appeared to be an increased risk of progression in patients with high-risk cytogenetics (HR 1.45; 95% CI: 1.05, 1.99; p=0.025) or those with
Summary
These data indicated that a fixed period (4 cycles) of BTZ consolidation was beneficial in NDMM patients with or without prior BTZ exposure. A higher proportion of patients achieved ≥VGPR after BTZ consolidation than OBS. PFS was significantly improved, but there was no improvement in OS at data cutoff, possibly related to limited follow-up and the use of effective salvage options. Subgroups that seemed to benefit from BTZ consolidation were patients with
Keyword(s): Bortezomib, Consolidation, Multiple myeloma, Phase III

Session topic: Multiple myeloma: Clinical studies 2
Abstract: S426
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:30 to 13.06.2015 11:45
Location: Room A2+3
Background
HDT followed by ASCT remains the gold standard for treatment of NDMM patients who are able to tolerate the procedure. Following ASCT, consolidation with novel agents for a fixed time period (or number of cycles) can improve outcomes. However, there are currently few published data on BTZ consolidation therapy, although a study by the Nordic Myeloma Study Group has reported results in BTZ-naïve patients (Mellqvist et al Blood 2013).
Aims
The results from two large randomized controlled phase 3 studies were combined to investigate BTZ consolidation or OBS in patients with NDMM both with and without prior BTZ treatment.
Methods
MMY3012 (NCT00416273; 222 patients aged ≤60 yrs) and MMY3013 (NCT00416208; 158 patients aged 61–75 yrs) recruited adults with NDMM who underwent induction therapy followed by ASCT. Patients were randomized 1:1 to receive BTZ consolidation (1.6 mg/m² IV days 1, 8, 15, 22; 4 x 35-day cycles) or OBS, 60–120 days after ASCT. The primary endpoint was progression-free survival (PFS) from the start of induction; secondary endpoints included response rate, overall survival (OS), and safety. Factors affecting PFS were also assessed by post-hoc multivariate analysis. Responses were assessed per EBMT response criteria, with VGPR as an additional category. Adverse events (AEs) were graded per NCI-CTCAE v3.0.
Results
In 371 randomized patients, median age was 59 yrs (35–76); 62% were male, 14% / 84% were Durie-Salmon stage II / III. Of the 278 patients assessed for cytogenetics, 37% were classified as high-risk (32% del13q, 10% t(4;14), 6% del17p). Overall, 50% of patients had received prior BTZ therapy; the most common induction regimen was VCD (40%). Others included dexamethasone/idarubicin (14%), dexamethasone (13%), VAD (9%), adriamycin/dexamethasone (6%) and VD (6%). In the overall study population, patients who received BTZ-based induction showed a trend towards improved PFS (HR 1.24; 95% CI: 0.97, 1.59; p=0.084) by unadjusted Cox regression analysis. Outcomes for patients receiving BTZ consolidation or OBS only are shown in the table (median follow-up 50 months from start of induction). PFS was significantly improved by approximately 6 months, but there was no improvement in OS. In a post-hoc exploratory multivariate analysis, BTZ consolidation remained a predictor for PFS (HR 0.69; 95% CI: 0.51, 0.93; p=0.016). There appeared to be an increased risk of progression in patients with high-risk cytogenetics (HR 1.45; 95% CI: 1.05, 1.99; p=0.025) or those with60 had no effect on PFS (HR 1.24; 95% CI: 0.87, 1.77; p=0.240); neither did the use of non-BTZ induction (HR 1.17; 95% CI: 0.83, 1.66; p=0.375).
Summary
These data indicated that a fixed period (4 cycles) of BTZ consolidation was beneficial in NDMM patients with or without prior BTZ exposure. A higher proportion of patients achieved ≥VGPR after BTZ consolidation than OBS. PFS was significantly improved, but there was no improvement in OS at data cutoff, possibly related to limited follow-up and the use of effective salvage options. Subgroups that seemed to benefit from BTZ consolidation were patients with
Keyword(s): Bortezomib, Consolidation, Multiple myeloma, Phase III

Session topic: Multiple myeloma: Clinical studies 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:30 to 13.06.2015 11:45
Location: Room A2+3
Background
HDT followed by ASCT remains the gold standard for treatment of NDMM patients who are able to tolerate the procedure. Following ASCT, consolidation with novel agents for a fixed time period (or number of cycles) can improve outcomes. However, there are currently few published data on BTZ consolidation therapy, although a study by the Nordic Myeloma Study Group has reported results in BTZ-naïve patients (Mellqvist et al Blood 2013).
Aims
The results from two large randomized controlled phase 3 studies were combined to investigate BTZ consolidation or OBS in patients with NDMM both with and without prior BTZ treatment.
Methods
MMY3012 (NCT00416273; 222 patients aged ≤60 yrs) and MMY3013 (NCT00416208; 158 patients aged 61–75 yrs) recruited adults with NDMM who underwent induction therapy followed by ASCT. Patients were randomized 1:1 to receive BTZ consolidation (1.6 mg/m² IV days 1, 8, 15, 22; 4 x 35-day cycles) or OBS, 60–120 days after ASCT. The primary endpoint was progression-free survival (PFS) from the start of induction; secondary endpoints included response rate, overall survival (OS), and safety. Factors affecting PFS were also assessed by post-hoc multivariate analysis. Responses were assessed per EBMT response criteria, with VGPR as an additional category. Adverse events (AEs) were graded per NCI-CTCAE v3.0.
Results
In 371 randomized patients, median age was 59 yrs (35–76); 62% were male, 14% / 84% were Durie-Salmon stage II / III. Of the 278 patients assessed for cytogenetics, 37% were classified as high-risk (32% del13q, 10% t(4;14), 6% del17p). Overall, 50% of patients had received prior BTZ therapy; the most common induction regimen was VCD (40%). Others included dexamethasone/idarubicin (14%), dexamethasone (13%), VAD (9%), adriamycin/dexamethasone (6%) and VD (6%). In the overall study population, patients who received BTZ-based induction showed a trend towards improved PFS (HR 1.24; 95% CI: 0.97, 1.59; p=0.084) by unadjusted Cox regression analysis. Outcomes for patients receiving BTZ consolidation or OBS only are shown in the table (median follow-up 50 months from start of induction). PFS was significantly improved by approximately 6 months, but there was no improvement in OS. In a post-hoc exploratory multivariate analysis, BTZ consolidation remained a predictor for PFS (HR 0.69; 95% CI: 0.51, 0.93; p=0.016). There appeared to be an increased risk of progression in patients with high-risk cytogenetics (HR 1.45; 95% CI: 1.05, 1.99; p=0.025) or those with
Summary
These data indicated that a fixed period (4 cycles) of BTZ consolidation was beneficial in NDMM patients with or without prior BTZ exposure. A higher proportion of patients achieved ≥VGPR after BTZ consolidation than OBS. PFS was significantly improved, but there was no improvement in OS at data cutoff, possibly related to limited follow-up and the use of effective salvage options. Subgroups that seemed to benefit from BTZ consolidation were patients with
Keyword(s): Bortezomib, Consolidation, Multiple myeloma, Phase III

Session topic: Multiple myeloma: Clinical studies 2
{{ help_message }}
{{filter}}


