
Contributions
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room Stolz 2
Background
Multiple myeloma (MM) is associated with high morbidity and mortality. In recent years, immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) have been approved for MM treatment based on their ability to improve patient (pt) survival. However, a greater understanding of quality of life (QoL) in patients treated with IMiDs and PIs is needed.
Aims
PREAMBLE (Prospective REsearch Assessment in Multiple Myeloma: an oBservationaL Evaluation; NCT01838512) is an ongoing, prospective, multinational, observational cohort study designed to better understand the real-world clinical effectiveness of IMiDs, PIs, and IMiD+PI in pts with relapsed/refractory MM (RRMM). We report QoL data for pts enrolled with ≥6 months’ follow-up.
Methods
PREAMBLE includes pts with RRMM aged ≥18 years who have received ≥1 prior therapy and who initiated treatment with an IMiD, PI, or IMiD+PI <90 days prior to or 30 days after enrollment. Administration is according to standard clinical practice. Data are collected at baseline (BL) and every 3 months during Year 1, then every 6 months in Years 2 and 3, or until discontinuation. Data extraction for the present analysis was performed on 12 December 2014. The EuroQol 5 Dimensions (EQ-5D) questionnaire and the European Organisation for Research and Treatment of Cancer (EORTC) QoL Questionnaire-Core 30 (QLQ-C30) were used to assess disease burden. Minimally important differences for EQ-5D and QLQ-C30 have been estimated at 0.06–0.09 (for all cancers)1 and 6–17 (for MM),2 respectively.
Results
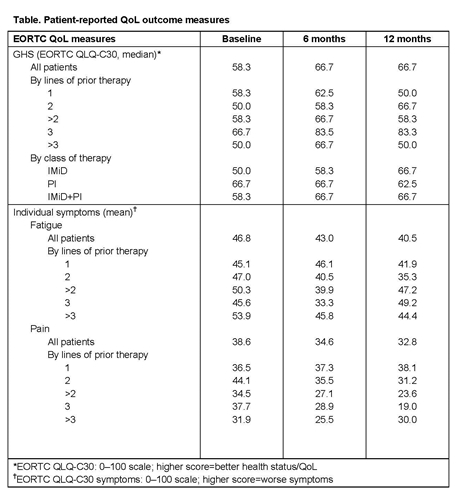
At the time of data extraction, 273 pts had ≥6 months’ follow-up (96% completion rate at BL). Median BL EQ-5D score was 0.69, compared with 0.8 in the general US population3 and 0.6–0.7 in other MM studies.4,5 At BL, there was no difference in median EQ-5D score regardless of number of prior lines of therapy. At Month 6, there was a trend towards improvement in median EQ-5D score with increasing numbers of prior lines of therapy (1: 0.69; 2: 0.69; >2: 0.76; 3: 0.76; >3: 0.76 ). At BL, pts reported some or severe pain and discomfort (63.4% [some] and 14.1% [severe]), anxiety and depression (45.5% [some] and 5.3% [severe]), and inability to conduct usual activities (52.9% [some] and 10.6% [severe]). More pts receiving IMiDs (19.4%) reported severe pain and discomfort at BL than those receiving PIs (8.3%) or IMiD+PI (11.1%). Median QLQ-C30 global health status (GHS) score at BL and Month 12 was 58.3 and 66.7, respectively, compared with 75 (general population), 66.7 (colorectal cancer pts), 66.7 (all cancer pts),6 and 57–68.7 (previous MM studies).4,5 Median QLQ-C30 GHS improved over time in pts treated with 3 prior lines of therapy or with IMiDs (Table; higher score=better QoL). At BL, fatigue and pain placed the greatest burden on pts, but improved over time (Table; higher score=worse symptoms).
Summary
Preliminary analysis suggests that MM continues to place a high burden on pts despite new therapies. Although functional scores show a high level of function, pts are particularly troubled by pain, discomfort, and fatigue. As data mature and more pts are enrolled, PREAMBLE will shed light on MM burden and its management.
References: 1. Pickard AS et al. Health Qual Life Outcomes 2007;5:70. 2. Kvam AK et al. Health Qual Life Outcomes 2010;8:79. 3. Szende A et al (eds). Self-Reported Population Health: An International Perspective Based on EQ-5D. Netherlands: Springer; 2014. 4. Acaster S et al. Support Care Cancer 2013;21:599–607. 5. Crott R et al. Qual Life Res 2013;22:1045–54. 6. Scott NW et al. EORTC QLQ-C30 Reference Values. Brussels: EORTC Quality of Life Group Publications; 2008
Keyword(s): Multiple myeloma, Quality of life
Session topic: Quality of life and health economics
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room Stolz 2
Background
Multiple myeloma (MM) is associated with high morbidity and mortality. In recent years, immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) have been approved for MM treatment based on their ability to improve patient (pt) survival. However, a greater understanding of quality of life (QoL) in patients treated with IMiDs and PIs is needed.
Aims
PREAMBLE (Prospective REsearch Assessment in Multiple Myeloma: an oBservationaL Evaluation; NCT01838512) is an ongoing, prospective, multinational, observational cohort study designed to better understand the real-world clinical effectiveness of IMiDs, PIs, and IMiD+PI in pts with relapsed/refractory MM (RRMM). We report QoL data for pts enrolled with ≥6 months’ follow-up.
Methods
PREAMBLE includes pts with RRMM aged ≥18 years who have received ≥1 prior therapy and who initiated treatment with an IMiD, PI, or IMiD+PI <90 days prior to or 30 days after enrollment. Administration is according to standard clinical practice. Data are collected at baseline (BL) and every 3 months during Year 1, then every 6 months in Years 2 and 3, or until discontinuation. Data extraction for the present analysis was performed on 12 December 2014. The EuroQol 5 Dimensions (EQ-5D) questionnaire and the European Organisation for Research and Treatment of Cancer (EORTC) QoL Questionnaire-Core 30 (QLQ-C30) were used to assess disease burden. Minimally important differences for EQ-5D and QLQ-C30 have been estimated at 0.06–0.09 (for all cancers)1 and 6–17 (for MM),2 respectively.
Results
At the time of data extraction, 273 pts had ≥6 months’ follow-up (96% completion rate at BL). Median BL EQ-5D score was 0.69, compared with 0.8 in the general US population3 and 0.6–0.7 in other MM studies.4,5 At BL, there was no difference in median EQ-5D score regardless of number of prior lines of therapy. At Month 6, there was a trend towards improvement in median EQ-5D score with increasing numbers of prior lines of therapy (1: 0.69; 2: 0.69; >2: 0.76; 3: 0.76; >3: 0.76 ). At BL, pts reported some or severe pain and discomfort (63.4% [some] and 14.1% [severe]), anxiety and depression (45.5% [some] and 5.3% [severe]), and inability to conduct usual activities (52.9% [some] and 10.6% [severe]). More pts receiving IMiDs (19.4%) reported severe pain and discomfort at BL than those receiving PIs (8.3%) or IMiD+PI (11.1%). Median QLQ-C30 global health status (GHS) score at BL and Month 12 was 58.3 and 66.7, respectively, compared with 75 (general population), 66.7 (colorectal cancer pts), 66.7 (all cancer pts),6 and 57–68.7 (previous MM studies).4,5 Median QLQ-C30 GHS improved over time in pts treated with 3 prior lines of therapy or with IMiDs (Table; higher score=better QoL). At BL, fatigue and pain placed the greatest burden on pts, but improved over time (Table; higher score=worse symptoms).
Summary
Preliminary analysis suggests that MM continues to place a high burden on pts despite new therapies. Although functional scores show a high level of function, pts are particularly troubled by pain, discomfort, and fatigue. As data mature and more pts are enrolled, PREAMBLE will shed light on MM burden and its management.
References: 1. Pickard AS et al. Health Qual Life Outcomes 2007;5:70. 2. Kvam AK et al. Health Qual Life Outcomes 2010;8:79. 3. Szende A et al (eds). Self-Reported Population Health: An International Perspective Based on EQ-5D. Netherlands: Springer; 2014. 4. Acaster S et al. Support Care Cancer 2013;21:599–607. 5. Crott R et al. Qual Life Res 2013;22:1045–54. 6. Scott NW et al. EORTC QLQ-C30 Reference Values. Brussels: EORTC Quality of Life Group Publications; 2008
Keyword(s): Multiple myeloma, Quality of life

Session topic: Quality of life and health economics


