Ca' Granda Foundation IRCCS

Contributions
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room Strauss 1
Background
Beta-thalassemias are characterized by ineffective erythropoiesis leading to anemia, iron overload, and organ failure. Sotatercept (ACE-011) is a novel and first-in-class activin type IIA receptor fusion protein that acts on late-stage erythropoiesis to increase the release of mature erythrocytes into circulation (Carrancio S, et al. Br J Haematol 2014;165:870-82). RAP-011, a murine version of sotatercept, was effective in a mouse model of beta-thalassemia, thereby supporting the clinical development of sotatercept for beta-thalassemia (Dussiot M, et al. Nat Med 2014;20:398-407).
Aims
To determine a safe, tolerable, and effective dose of sotatercept in pts with transfusion-dependent (TD) beta-thalassemia major (TM) and pts with TD or non-transfusion-dependent (NTD) beta-thalassemia intermedia (TI).
Methods
In this ongoing phase 2a, multicenter, open-label, dose-finding study, pts received sotatercept subcutaneously once every 3 weeks; enrollment up to 1.0 mg/kg is complete and analysis ongoing. Efficacy was assessed by hemoglobin (Hb) increase from baseline for NTD pts and RBC transfusion burden reduction for TD pts. Safety was assessed by NCI-CTCAE v4.0. All pts provided informed consent. ClinicalTrials.gov identifier: NCT01571635.
Results
30 of 46 pts (65%) in the sotatercept 0.1, 0.3, 0.5, 0.75, and 1.0 mg/kg dose groups were NTD (28 TI, 2 Hb-E/beta-thalassemia) and 16 (35%) were TD (12 TM, 4 TI).
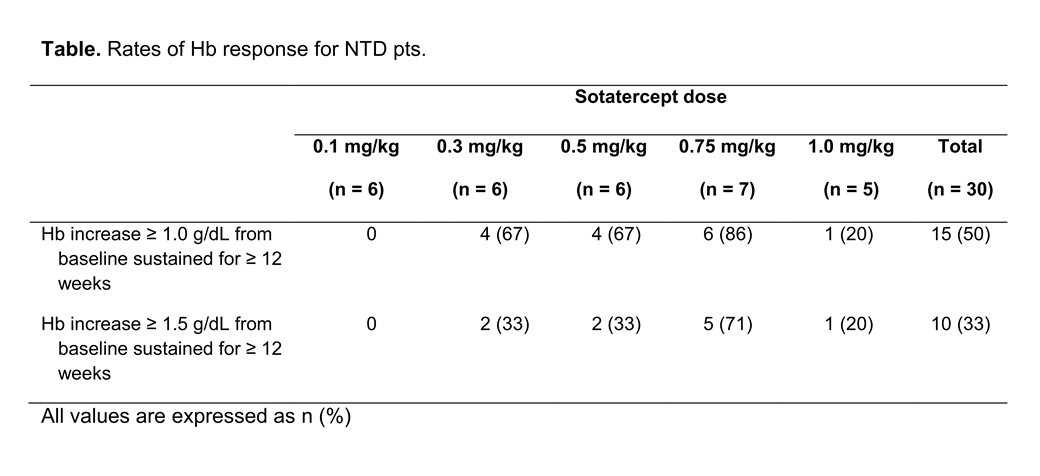
Of 30 NTD pts, 6, 6, 6, 7, and 5 were included in the different dose groups, respectively; mean baseline Hb for NTD pts was 8.3 g/dL (range 5.9–10.7). Rates of Hb response for NTD pts are shown in the Table.
Area under the curve and peak concentration of sotatercept increased with dose (data not shown). Increased exposure was associated with higher mean Hb increases over 9 weeks for NTD pts (r = 0.78, P < 0.0001) and with reduced transfusion burden over 24 weeks for TD pts (r = 0.74, P < 0.01).
Among TD pts evaluable for efficacy, 8 of 14 pts (57%) showed a ≥ 20% reduction in transfusion burden on treatment versus 6 month transfusion burden at baseline, whereas ≥ 50% reduction was observed for 1 of 5 pts in the 0.75 mg/kg dose group and 1 of 2 pts in the 1.0 mg/kg dose group; treatment of the 0.3, 0.5, 0.75, and 1.0 mg/kg groups is ongoing.
Overall, sotatercept was well tolerated and 25 of 46 pts (54%) remain on treatment; 19 (41%) pts have been on treatment for ≥ 1 year, 8 (17%) for ≥ 22 months, and 2 (4%) for ≥ 2 years. Grade ≥ 3 treatment-related adverse events leading to discontinuation were seen in 4 pts: worsening grade 3 bone pain in 1 TD pt in the 0.1 mg/kg group; grade 3 ventricular extrasystoles in 1 NTD pt in the 0.5 mg/kg group; and grade 3 hypertension in 2 NTD pts in the 0.75 mg/kg group. Overall, 3 pts discontinued due to lack of efficacy: 1 pt in each of the 0.1, 0.3, and 1.0 mg/kg dose groups. Updated safety and efficacy data will be presented.
Summary
These preliminary data suggest long-term treatment with sotatercept can increase Hb levels and decrease transfusion burden with a favorable safety profile in pts with beta-thalassemia. These data suggest an exposure-dependent effect for sotatercept.
MDC, JP, and OH contributed equally to this abstract.
Keyword(s): Anemia, Beta thalassemia, Clinical trial, Erythropoieisis
Session topic: Red cells: Novel clinical aspects
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room Strauss 1
Background
Beta-thalassemias are characterized by ineffective erythropoiesis leading to anemia, iron overload, and organ failure. Sotatercept (ACE-011) is a novel and first-in-class activin type IIA receptor fusion protein that acts on late-stage erythropoiesis to increase the release of mature erythrocytes into circulation (Carrancio S, et al. Br J Haematol 2014;165:870-82). RAP-011, a murine version of sotatercept, was effective in a mouse model of beta-thalassemia, thereby supporting the clinical development of sotatercept for beta-thalassemia (Dussiot M, et al. Nat Med 2014;20:398-407).
Aims
To determine a safe, tolerable, and effective dose of sotatercept in pts with transfusion-dependent (TD) beta-thalassemia major (TM) and pts with TD or non-transfusion-dependent (NTD) beta-thalassemia intermedia (TI).
Methods
In this ongoing phase 2a, multicenter, open-label, dose-finding study, pts received sotatercept subcutaneously once every 3 weeks; enrollment up to 1.0 mg/kg is complete and analysis ongoing. Efficacy was assessed by hemoglobin (Hb) increase from baseline for NTD pts and RBC transfusion burden reduction for TD pts. Safety was assessed by NCI-CTCAE v4.0. All pts provided informed consent. ClinicalTrials.gov identifier: NCT01571635.
Results
30 of 46 pts (65%) in the sotatercept 0.1, 0.3, 0.5, 0.75, and 1.0 mg/kg dose groups were NTD (28 TI, 2 Hb-E/beta-thalassemia) and 16 (35%) were TD (12 TM, 4 TI).
Of 30 NTD pts, 6, 6, 6, 7, and 5 were included in the different dose groups, respectively; mean baseline Hb for NTD pts was 8.3 g/dL (range 5.9–10.7). Rates of Hb response for NTD pts are shown in the Table.
Area under the curve and peak concentration of sotatercept increased with dose (data not shown). Increased exposure was associated with higher mean Hb increases over 9 weeks for NTD pts (r = 0.78, P < 0.0001) and with reduced transfusion burden over 24 weeks for TD pts (r = 0.74, P < 0.01).
Among TD pts evaluable for efficacy, 8 of 14 pts (57%) showed a ≥ 20% reduction in transfusion burden on treatment versus 6 month transfusion burden at baseline, whereas ≥ 50% reduction was observed for 1 of 5 pts in the 0.75 mg/kg dose group and 1 of 2 pts in the 1.0 mg/kg dose group; treatment of the 0.3, 0.5, 0.75, and 1.0 mg/kg groups is ongoing.
Overall, sotatercept was well tolerated and 25 of 46 pts (54%) remain on treatment; 19 (41%) pts have been on treatment for ≥ 1 year, 8 (17%) for ≥ 22 months, and 2 (4%) for ≥ 2 years. Grade ≥ 3 treatment-related adverse events leading to discontinuation were seen in 4 pts: worsening grade 3 bone pain in 1 TD pt in the 0.1 mg/kg group; grade 3 ventricular extrasystoles in 1 NTD pt in the 0.5 mg/kg group; and grade 3 hypertension in 2 NTD pts in the 0.75 mg/kg group. Overall, 3 pts discontinued due to lack of efficacy: 1 pt in each of the 0.1, 0.3, and 1.0 mg/kg dose groups. Updated safety and efficacy data will be presented.
Summary
These preliminary data suggest long-term treatment with sotatercept can increase Hb levels and decrease transfusion burden with a favorable safety profile in pts with beta-thalassemia. These data suggest an exposure-dependent effect for sotatercept.
MDC, JP, and OH contributed equally to this abstract.
Keyword(s): Anemia, Beta thalassemia, Clinical trial, Erythropoieisis

Session topic: Red cells: Novel clinical aspects


