EFFECT OF AGE ON EFFICACY AND SAFETY OUTCOMES IN PATIENTS WITH NEWLY DIAGNOSED MULTIPLE MYELOMA RECEIVING LENALIDOMIDE AND LOW-DOSE DEXAMETHASONE (RD): THE FIRST TRIAL
(Abstract release date: 05/21/15)
EHA Library. Hulin C. 06/13/15; 103067; S429

Cyrille Hulin
Contributions
Contributions
Abstract
Abstract: S429
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:15 to 13.06.2015 12:30
Location: Room A2+3
Background
Combination therapy with melphalan-prednisone-thalidomide (MPT) is considered a standard treatment (Tx) option for patients (pts) with newly diagnosed multiple myeloma (NDMM) who are ineligible for stem cell transplant (SCT; Facon, Lancet Oncol, 2007; Fayers, Blood, 2011; NCCN Guidelines, Multiple Myeloma, V3.2015). The pivotal, randomized, international, multicenter phase 3 FIRST trial demonstrated that first-line use of lenalidomide continuous plus low-dose dexamethasone (Rd continuous) improved progression-free survival (PFS) compared with MPT (HR = 0.72; P < 0.001). An overall survival (OS) analysis also showed improvement, with a 22% reduction in risk of death with Rd continuous vs MPT (HR = 0.78; P = 0.02) (Benboubker, N Engl J Med, 2014).
Aims
To determine PFS, OS, and DOR outcomes stratified by age of patients with NDMM in the FIRST trial.
Methods
NDMM pts ineligible for SCT were randomized 1:1:1 to Tx with Rd continuous (28-day cycles) until disease progression (n = 535); 18 cycles (72 wks) of Rd (Rd18; n = 541); or 12 cycles
(72 wks) of MPT (n = 547). Starting doses were reduced in pts aged > 75 vs those aged ≤ 75 yrs: dexamethasone (20 vs 40 mg), melphalan (0.20 vs 0.25 mg/kg), and thalidomide (100 vs 200 mg). The primary endpoint was PFS. Secondary endpoints included OS, overall response rate (ORR), time to response, duration of response (DOR), time to Tx failure, time to second anti-myeloma Tx, health-related quality of life, and safety.
Results
This analysis based on the final PFS analysis using a data cutoff of May 24, 2013, with a median follow-up of 37 mos. The proportion of pts aged ≤ 75 yrs and > 75 yrs was 65% (n = 1056) and 35%
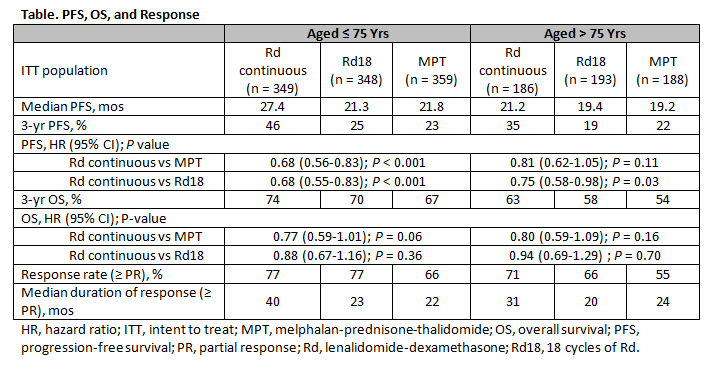
(n = 567), respectively. Pt characteristics, including rate of adverse cytogenetics, were well balanced across all Tx arms. In pts ≤ 75 yrs, ISS stage III disease was detected in 37% of those treated with Rd continuous or Rd18 and 36% of pts treated with MPT. In pts > 75 yrs, these rates were 47% with Rd continuous or Rd18 and 50% with MPT. Severe renal impairment (CrCl < 30 mL/min) was observed in 6%, 8%, and 8% of pts ≤ 75 yrs vs 13%, 11%, and 15% of pts > 75 yrs (Rd continuous, Rd18, and MPT, respectively). PFS and OS outcomes favored Rd continuous over MPT in both age groups. Median PFS was 27.4 mos in Rd continuous pts vs 21.8 mos in MPT pts aged ≤ 75 yrs (HR = 0.68; P < 0.001); HR for pts aged > 75 yrs was 0.81 (P = 0.11; Table). PFS for Rd continuous vs Rd18 pts was also increased in both age groups (HR = 0.68; P < 0.001 and HR = 0.75; P = 0.03, respectively). OS showed an improved trend for Rd continuous vs MPT in pts aged ≤ 75 yrs (HR = 0.77; P = 0.06) and > 75 yrs (HR = 0.80; P = 0.16). ORR was consistently higher with Rd continuous vs MPT in pts aged ≤ 75 yrs (77% vs 66%) and > 75 yrs (71% vs 55%). Median DOR with Rd continuous was longer vs MPT in pts aged ≤ 75 yrs (40 vs 22 mos) and pts > 75 yrs (31 vs 24 mos). Hematologic and non-hematologic adverse events (AEs) were as expected for Rd and MPT, with no relevant differences in Grade 3/4 toxicity between pts ≤ 75 yrs and > 75 yrs. Tx discontinuation due to AEs was comparable across Tx and age groups.
Summary
Regardless of age (≤ 75 vs > 75 yrs), Rd continuous extended PFS with an OS benefit vs MPT in NDMM pts. Rd continuous was generally well tolerated in both age groups. DOR was improved with Rd continuous vs MPT and Rd18, irrespective of age. Rd continuous represents a new standard of care for pts in the first-line setting independent of age.
Keyword(s): Age, Elderly, Multiple myeloma
Session topic: Multiple myeloma: Clinical studies 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:15 to 13.06.2015 12:30
Location: Room A2+3
Background
Combination therapy with melphalan-prednisone-thalidomide (MPT) is considered a standard treatment (Tx) option for patients (pts) with newly diagnosed multiple myeloma (NDMM) who are ineligible for stem cell transplant (SCT; Facon, Lancet Oncol, 2007; Fayers, Blood, 2011; NCCN Guidelines, Multiple Myeloma, V3.2015). The pivotal, randomized, international, multicenter phase 3 FIRST trial demonstrated that first-line use of lenalidomide continuous plus low-dose dexamethasone (Rd continuous) improved progression-free survival (PFS) compared with MPT (HR = 0.72; P < 0.001). An overall survival (OS) analysis also showed improvement, with a 22% reduction in risk of death with Rd continuous vs MPT (HR = 0.78; P = 0.02) (Benboubker, N Engl J Med, 2014).
Aims
To determine PFS, OS, and DOR outcomes stratified by age of patients with NDMM in the FIRST trial.
Methods
NDMM pts ineligible for SCT were randomized 1:1:1 to Tx with Rd continuous (28-day cycles) until disease progression (n = 535); 18 cycles (72 wks) of Rd (Rd18; n = 541); or 12 cycles
(72 wks) of MPT (n = 547). Starting doses were reduced in pts aged > 75 vs those aged ≤ 75 yrs: dexamethasone (20 vs 40 mg), melphalan (0.20 vs 0.25 mg/kg), and thalidomide (100 vs 200 mg). The primary endpoint was PFS. Secondary endpoints included OS, overall response rate (ORR), time to response, duration of response (DOR), time to Tx failure, time to second anti-myeloma Tx, health-related quality of life, and safety.
Results
This analysis based on the final PFS analysis using a data cutoff of May 24, 2013, with a median follow-up of 37 mos. The proportion of pts aged ≤ 75 yrs and > 75 yrs was 65% (n = 1056) and 35%
(n = 567), respectively. Pt characteristics, including rate of adverse cytogenetics, were well balanced across all Tx arms. In pts ≤ 75 yrs, ISS stage III disease was detected in 37% of those treated with Rd continuous or Rd18 and 36% of pts treated with MPT. In pts > 75 yrs, these rates were 47% with Rd continuous or Rd18 and 50% with MPT. Severe renal impairment (CrCl < 30 mL/min) was observed in 6%, 8%, and 8% of pts ≤ 75 yrs vs 13%, 11%, and 15% of pts > 75 yrs (Rd continuous, Rd18, and MPT, respectively). PFS and OS outcomes favored Rd continuous over MPT in both age groups. Median PFS was 27.4 mos in Rd continuous pts vs 21.8 mos in MPT pts aged ≤ 75 yrs (HR = 0.68; P < 0.001); HR for pts aged > 75 yrs was 0.81 (P = 0.11; Table). PFS for Rd continuous vs Rd18 pts was also increased in both age groups (HR = 0.68; P < 0.001 and HR = 0.75; P = 0.03, respectively). OS showed an improved trend for Rd continuous vs MPT in pts aged ≤ 75 yrs (HR = 0.77; P = 0.06) and > 75 yrs (HR = 0.80; P = 0.16). ORR was consistently higher with Rd continuous vs MPT in pts aged ≤ 75 yrs (77% vs 66%) and > 75 yrs (71% vs 55%). Median DOR with Rd continuous was longer vs MPT in pts aged ≤ 75 yrs (40 vs 22 mos) and pts > 75 yrs (31 vs 24 mos). Hematologic and non-hematologic adverse events (AEs) were as expected for Rd and MPT, with no relevant differences in Grade 3/4 toxicity between pts ≤ 75 yrs and > 75 yrs. Tx discontinuation due to AEs was comparable across Tx and age groups.
Summary
Regardless of age (≤ 75 vs > 75 yrs), Rd continuous extended PFS with an OS benefit vs MPT in NDMM pts. Rd continuous was generally well tolerated in both age groups. DOR was improved with Rd continuous vs MPT and Rd18, irrespective of age. Rd continuous represents a new standard of care for pts in the first-line setting independent of age.
Keyword(s): Age, Elderly, Multiple myeloma

Session topic: Multiple myeloma: Clinical studies 2
Abstract: S429
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:15 to 13.06.2015 12:30
Location: Room A2+3
Background
Combination therapy with melphalan-prednisone-thalidomide (MPT) is considered a standard treatment (Tx) option for patients (pts) with newly diagnosed multiple myeloma (NDMM) who are ineligible for stem cell transplant (SCT; Facon, Lancet Oncol, 2007; Fayers, Blood, 2011; NCCN Guidelines, Multiple Myeloma, V3.2015). The pivotal, randomized, international, multicenter phase 3 FIRST trial demonstrated that first-line use of lenalidomide continuous plus low-dose dexamethasone (Rd continuous) improved progression-free survival (PFS) compared with MPT (HR = 0.72; P < 0.001). An overall survival (OS) analysis also showed improvement, with a 22% reduction in risk of death with Rd continuous vs MPT (HR = 0.78; P = 0.02) (Benboubker, N Engl J Med, 2014).
Aims
To determine PFS, OS, and DOR outcomes stratified by age of patients with NDMM in the FIRST trial.
Methods
NDMM pts ineligible for SCT were randomized 1:1:1 to Tx with Rd continuous (28-day cycles) until disease progression (n = 535); 18 cycles (72 wks) of Rd (Rd18; n = 541); or 12 cycles
(72 wks) of MPT (n = 547). Starting doses were reduced in pts aged > 75 vs those aged ≤ 75 yrs: dexamethasone (20 vs 40 mg), melphalan (0.20 vs 0.25 mg/kg), and thalidomide (100 vs 200 mg). The primary endpoint was PFS. Secondary endpoints included OS, overall response rate (ORR), time to response, duration of response (DOR), time to Tx failure, time to second anti-myeloma Tx, health-related quality of life, and safety.
Results
This analysis based on the final PFS analysis using a data cutoff of May 24, 2013, with a median follow-up of 37 mos. The proportion of pts aged ≤ 75 yrs and > 75 yrs was 65% (n = 1056) and 35%
(n = 567), respectively. Pt characteristics, including rate of adverse cytogenetics, were well balanced across all Tx arms. In pts ≤ 75 yrs, ISS stage III disease was detected in 37% of those treated with Rd continuous or Rd18 and 36% of pts treated with MPT. In pts > 75 yrs, these rates were 47% with Rd continuous or Rd18 and 50% with MPT. Severe renal impairment (CrCl < 30 mL/min) was observed in 6%, 8%, and 8% of pts ≤ 75 yrs vs 13%, 11%, and 15% of pts > 75 yrs (Rd continuous, Rd18, and MPT, respectively). PFS and OS outcomes favored Rd continuous over MPT in both age groups. Median PFS was 27.4 mos in Rd continuous pts vs 21.8 mos in MPT pts aged ≤ 75 yrs (HR = 0.68; P < 0.001); HR for pts aged > 75 yrs was 0.81 (P = 0.11; Table). PFS for Rd continuous vs Rd18 pts was also increased in both age groups (HR = 0.68; P < 0.001 and HR = 0.75; P = 0.03, respectively). OS showed an improved trend for Rd continuous vs MPT in pts aged ≤ 75 yrs (HR = 0.77; P = 0.06) and > 75 yrs (HR = 0.80; P = 0.16). ORR was consistently higher with Rd continuous vs MPT in pts aged ≤ 75 yrs (77% vs 66%) and > 75 yrs (71% vs 55%). Median DOR with Rd continuous was longer vs MPT in pts aged ≤ 75 yrs (40 vs 22 mos) and pts > 75 yrs (31 vs 24 mos). Hematologic and non-hematologic adverse events (AEs) were as expected for Rd and MPT, with no relevant differences in Grade 3/4 toxicity between pts ≤ 75 yrs and > 75 yrs. Tx discontinuation due to AEs was comparable across Tx and age groups.
Summary
Regardless of age (≤ 75 vs > 75 yrs), Rd continuous extended PFS with an OS benefit vs MPT in NDMM pts. Rd continuous was generally well tolerated in both age groups. DOR was improved with Rd continuous vs MPT and Rd18, irrespective of age. Rd continuous represents a new standard of care for pts in the first-line setting independent of age.
Keyword(s): Age, Elderly, Multiple myeloma

Session topic: Multiple myeloma: Clinical studies 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:15 to 13.06.2015 12:30
Location: Room A2+3
Background
Combination therapy with melphalan-prednisone-thalidomide (MPT) is considered a standard treatment (Tx) option for patients (pts) with newly diagnosed multiple myeloma (NDMM) who are ineligible for stem cell transplant (SCT; Facon, Lancet Oncol, 2007; Fayers, Blood, 2011; NCCN Guidelines, Multiple Myeloma, V3.2015). The pivotal, randomized, international, multicenter phase 3 FIRST trial demonstrated that first-line use of lenalidomide continuous plus low-dose dexamethasone (Rd continuous) improved progression-free survival (PFS) compared with MPT (HR = 0.72; P < 0.001). An overall survival (OS) analysis also showed improvement, with a 22% reduction in risk of death with Rd continuous vs MPT (HR = 0.78; P = 0.02) (Benboubker, N Engl J Med, 2014).
Aims
To determine PFS, OS, and DOR outcomes stratified by age of patients with NDMM in the FIRST trial.
Methods
NDMM pts ineligible for SCT were randomized 1:1:1 to Tx with Rd continuous (28-day cycles) until disease progression (n = 535); 18 cycles (72 wks) of Rd (Rd18; n = 541); or 12 cycles
(72 wks) of MPT (n = 547). Starting doses were reduced in pts aged > 75 vs those aged ≤ 75 yrs: dexamethasone (20 vs 40 mg), melphalan (0.20 vs 0.25 mg/kg), and thalidomide (100 vs 200 mg). The primary endpoint was PFS. Secondary endpoints included OS, overall response rate (ORR), time to response, duration of response (DOR), time to Tx failure, time to second anti-myeloma Tx, health-related quality of life, and safety.
Results
This analysis based on the final PFS analysis using a data cutoff of May 24, 2013, with a median follow-up of 37 mos. The proportion of pts aged ≤ 75 yrs and > 75 yrs was 65% (n = 1056) and 35%
(n = 567), respectively. Pt characteristics, including rate of adverse cytogenetics, were well balanced across all Tx arms. In pts ≤ 75 yrs, ISS stage III disease was detected in 37% of those treated with Rd continuous or Rd18 and 36% of pts treated with MPT. In pts > 75 yrs, these rates were 47% with Rd continuous or Rd18 and 50% with MPT. Severe renal impairment (CrCl < 30 mL/min) was observed in 6%, 8%, and 8% of pts ≤ 75 yrs vs 13%, 11%, and 15% of pts > 75 yrs (Rd continuous, Rd18, and MPT, respectively). PFS and OS outcomes favored Rd continuous over MPT in both age groups. Median PFS was 27.4 mos in Rd continuous pts vs 21.8 mos in MPT pts aged ≤ 75 yrs (HR = 0.68; P < 0.001); HR for pts aged > 75 yrs was 0.81 (P = 0.11; Table). PFS for Rd continuous vs Rd18 pts was also increased in both age groups (HR = 0.68; P < 0.001 and HR = 0.75; P = 0.03, respectively). OS showed an improved trend for Rd continuous vs MPT in pts aged ≤ 75 yrs (HR = 0.77; P = 0.06) and > 75 yrs (HR = 0.80; P = 0.16). ORR was consistently higher with Rd continuous vs MPT in pts aged ≤ 75 yrs (77% vs 66%) and > 75 yrs (71% vs 55%). Median DOR with Rd continuous was longer vs MPT in pts aged ≤ 75 yrs (40 vs 22 mos) and pts > 75 yrs (31 vs 24 mos). Hematologic and non-hematologic adverse events (AEs) were as expected for Rd and MPT, with no relevant differences in Grade 3/4 toxicity between pts ≤ 75 yrs and > 75 yrs. Tx discontinuation due to AEs was comparable across Tx and age groups.
Summary
Regardless of age (≤ 75 vs > 75 yrs), Rd continuous extended PFS with an OS benefit vs MPT in NDMM pts. Rd continuous was generally well tolerated in both age groups. DOR was improved with Rd continuous vs MPT and Rd18, irrespective of age. Rd continuous represents a new standard of care for pts in the first-line setting independent of age.
Keyword(s): Age, Elderly, Multiple myeloma

Session topic: Multiple myeloma: Clinical studies 2
{{ help_message }}
{{filter}}


