
Contributions
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:45 to 13.06.2015 17:00
Location: Room Stolz 1
Background
EPAG, an oral thrombopoietin receptor agonist (TPO-RA), is approved for treating thrombocytopenia in adults with chronic immune thrombocytopenia (cITP) with insufficient response to prior therapy. TPO-RAs are associated with varying degrees of BM reticulin increases.1 Due to lack of pretreatment evaluations, the incidence and clinical significance of these findings have not been established. Inconsistencies in specimen preparation, staining, and analysis across institutions further confound conclusions.
Aims
To assess the degree of BM fibrosis (reticulin and/or collagen) in cITP patients (pts) treated for up to 2 years with EPAG.
Methods
Informed consent was provided. BM biopsies were collected at baseline (prior to study treatment) and at 1 and 2 years on treatment. Specimens were centrally processed, stained for reticulin (silver) and collagen (trichrome), and reviewed by central independent hematopathologists for cellularity; megakaryocyte, erythroid, and myeloid quantity and appearance; trabecular bone quality; reticulin grade (European Consensus scale of marrow fibrosis [MF]2); and presence of collagen.
Results
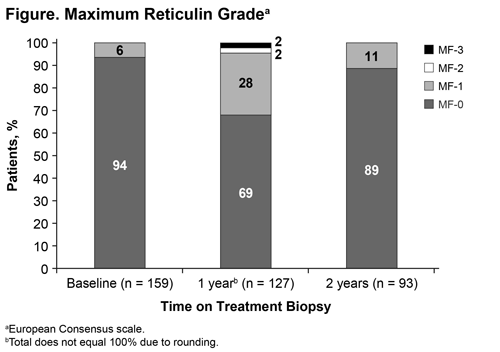
Of 162 pts analyzed, the median age was 42 years (range, 18–80), 104 pts (64%) were female, 50% were White, 20% East Asian, and 29% South Central Asian. A total of 77% were diagnosed with ITP ≥12 months before study entry. Prior ITP therapy was reported by 70%, and 8% received prior TPO-RA treatment. Of 162 pts, 44 withdrew from the study, 118 completed the study, and 93 had all 3 on-treatment biopsies. MF grades at each time point are shown in the Figure. Of the 3 pts with MF-2 at 1 year, 1 completed 2 years of treatment and had MF-2 at 22 days post-treatment and MF-1 at 288 days post-treatment. Another pt received 17 months EPAG and had MF-0 at 44 days post-treatment. The third pt did not have a follow-up biopsy. Of the 2 pts with MF-3, 1 had an on-treatment MF-0 biopsy 31 days after the MF-3 biopsy and at 2 years and 1 had MF-0 at 184 days post-treatment. At 2 years, no pt on treatment had ≥MF-2. When comparing biopsies at 2 years vs baseline, 80 pts (86%) had no change in MF grade, 9 (10%) had a 1-grade increase from MF-0 to MF-1, and 2 (2%) had a 1-grade decrease from MF-1 to MF-0.
All pts were negative for collagen at baseline. At 1 year, 5 pts (4%) were positive for collagen, 3 of whom had MF-2 or MF-3. At 2 years, 1 pt (1%) with MF-1 had collagen present. None of the on-treatment biopsies were prompted by an abnormal peripheral blood smear or done at the investigator’s discretion for clinical symptoms suggestive of BM dysfunction. Cellularity was normal in 80%, 80%, and 76% of pts at baseline, 1 year, and 2 years, respectively. Trabecular bone thinning was found in 73 pts (50%) at baseline, 61 (50%) at 1 year, and 33 (38%) at 2 years, likely the result of prior steroid therapy.
Summary
After 2 years of treatment, no increase in reticulin was observed in the majority of pts (86%) while few pts (10%) had a mild increase in reticulin. No pt had an on-treatment biopsy of ≥MF-2 at 2 years or clinical signs/symptoms indicative of BM dysfunction. Results were similar to those reported for EXTEND, an EPAG extension study (treatment duration up to 5.5 years3). These data suggest that treatment with EPAG is generally not associated with clinically relevant increases in BM reticulin or collagen. This study (NCT01098487) was funded by GlaxoSmithKline.
1. Ghanima W, et al. Haematologica. 2014;99:937–944.
2. Thiele J, et al. Haematologica. 2005;90:1128–1132.
Keyword(s): Bone Marrow Fibrosis, Immune thrombocytopenia (ITP), Therapy
Session topic: Platelet and bleeding disorders
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:45 to 13.06.2015 17:00
Location: Room Stolz 1
Background
EPAG, an oral thrombopoietin receptor agonist (TPO-RA), is approved for treating thrombocytopenia in adults with chronic immune thrombocytopenia (cITP) with insufficient response to prior therapy. TPO-RAs are associated with varying degrees of BM reticulin increases.1 Due to lack of pretreatment evaluations, the incidence and clinical significance of these findings have not been established. Inconsistencies in specimen preparation, staining, and analysis across institutions further confound conclusions.
Aims
To assess the degree of BM fibrosis (reticulin and/or collagen) in cITP patients (pts) treated for up to 2 years with EPAG.
Methods
Informed consent was provided. BM biopsies were collected at baseline (prior to study treatment) and at 1 and 2 years on treatment. Specimens were centrally processed, stained for reticulin (silver) and collagen (trichrome), and reviewed by central independent hematopathologists for cellularity; megakaryocyte, erythroid, and myeloid quantity and appearance; trabecular bone quality; reticulin grade (European Consensus scale of marrow fibrosis [MF]2); and presence of collagen.
Results
Of 162 pts analyzed, the median age was 42 years (range, 18–80), 104 pts (64%) were female, 50% were White, 20% East Asian, and 29% South Central Asian. A total of 77% were diagnosed with ITP ≥12 months before study entry. Prior ITP therapy was reported by 70%, and 8% received prior TPO-RA treatment. Of 162 pts, 44 withdrew from the study, 118 completed the study, and 93 had all 3 on-treatment biopsies. MF grades at each time point are shown in the Figure. Of the 3 pts with MF-2 at 1 year, 1 completed 2 years of treatment and had MF-2 at 22 days post-treatment and MF-1 at 288 days post-treatment. Another pt received 17 months EPAG and had MF-0 at 44 days post-treatment. The third pt did not have a follow-up biopsy. Of the 2 pts with MF-3, 1 had an on-treatment MF-0 biopsy 31 days after the MF-3 biopsy and at 2 years and 1 had MF-0 at 184 days post-treatment. At 2 years, no pt on treatment had ≥MF-2. When comparing biopsies at 2 years vs baseline, 80 pts (86%) had no change in MF grade, 9 (10%) had a 1-grade increase from MF-0 to MF-1, and 2 (2%) had a 1-grade decrease from MF-1 to MF-0.
All pts were negative for collagen at baseline. At 1 year, 5 pts (4%) were positive for collagen, 3 of whom had MF-2 or MF-3. At 2 years, 1 pt (1%) with MF-1 had collagen present. None of the on-treatment biopsies were prompted by an abnormal peripheral blood smear or done at the investigator’s discretion for clinical symptoms suggestive of BM dysfunction. Cellularity was normal in 80%, 80%, and 76% of pts at baseline, 1 year, and 2 years, respectively. Trabecular bone thinning was found in 73 pts (50%) at baseline, 61 (50%) at 1 year, and 33 (38%) at 2 years, likely the result of prior steroid therapy.
Summary
After 2 years of treatment, no increase in reticulin was observed in the majority of pts (86%) while few pts (10%) had a mild increase in reticulin. No pt had an on-treatment biopsy of ≥MF-2 at 2 years or clinical signs/symptoms indicative of BM dysfunction. Results were similar to those reported for EXTEND, an EPAG extension study (treatment duration up to 5.5 years3). These data suggest that treatment with EPAG is generally not associated with clinically relevant increases in BM reticulin or collagen. This study (NCT01098487) was funded by GlaxoSmithKline.
1. Ghanima W, et al. Haematologica. 2014;99:937–944.
2. Thiele J, et al. Haematologica. 2005;90:1128–1132.
Keyword(s): Bone Marrow Fibrosis, Immune thrombocytopenia (ITP), Therapy

Session topic: Platelet and bleeding disorders


