PHASE 1 MULTIPLE ASCENDING DOSE STUDY OF THE SAFETY, TOLERABILITY, AND PHARMACOKINETICS/PHARMACODYNAMICS OF AG-348, A FIRST-IN-CLASS ALLOSTERIC ACTIVATOR OF PYRUVATE KINASE-R, IN HEALTHY SUBJECTS
(Abstract release date: 05/21/15)
EHA Library. Yang H. 06/12/15; 103061; S138

Hua Yang
Contributions
Contributions
Abstract
Abstract: S138
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room Strauss 1
Background
Pyruvate kinase (PK) deficiency is a rare genetic disorder of metabolism resulting in chronic hemolytic anemia, with comorbidities including iron overload and multi-organ dysfunction. Approximately 180 mutations in the PKLR gene are recognized to cause functional deficiency of the PK-R isoform. Defective glycolysis in red blood cells leads to increases in the metabolic precursor, 2,3-diphosphoglycerate (2,3-DPG), and deficiency of the product, adenosine triphosphate (ATP). AG-348 is an oral allosteric activator of both wild type and multiple mutant PK-Rs. We report here a multiple ascending dose (MAD) trial of AG-348.
Aims
To assess safety, tolerability, and pharmacokinetics/pharmacodynamics (PK/PD) of AG-348 in healthy volunteers and identify a dosing schedule for future trials in patients with PK deficiency.
Methods
A phase 1, single-center, randomized, double-blind, placebo-controlled MAD study (NCT02149966) was conducted in healthy men and women (18–60 years) in 6 sequential cohorts (each cohort: n=6 AG-348, n=2 placebo [P]). Subjects gave informed consent and received oral AG-348 at 15–700 mg twice daily (q12h) or 120 mg once daily (q24h) for 14 days with follow-up to Day 29. Adverse events (AEs), laboratory parameters, ECGs, and vital signs were monitored. Plasma AG-348 concentrations and whole blood 2,3-DPG and ATP levels were measured in serial blood samples for PK/PD assessment. Hormone levels were monitored due to pre-clinical data suggesting potential modulation.
Results
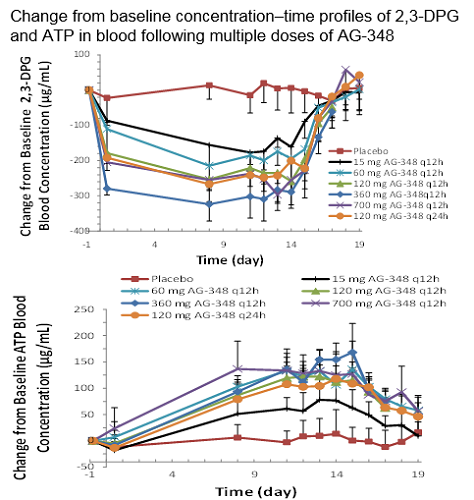
48 subjects (42M/6F), mean age 41.5 (range, 25–60) years, enrolled. Final, un-blinded safety data showed ≥1 AE in 16/36 (44%) AG-348-treated and 4/12 (33%) P subjects. Treatment-related AEs (≥1) were noted in 11/36 (31%) AG-348-treated and 3/12 (25%) P subjects. All treatment-related AEs were mild or moderate in severity (only one grade 3 event) and often reversible despite continued dosing. The most frequent AG-348-related AEs were nausea and headache, 5/36 (14%) for each (P: nausea 0/12 [0%]; headache 1/12 [8%]). Gastrointestinal AEs occurred in AG-348-treated subjects only at the highest dose, 700 mg q12h. One grade 3 AE occurred (AG-348 700 mg q12h, elevated liver function tests [LFTs], which resolved after treatment discontinuation). There were 4 AG-348 discontinuations: due to AEs in 2 subjects (grade 2 drug eruption, 60 mg q12h; grade 3 elevated LFTs, 700 mg q12h), and 2 subjects withdrew consent (both had grade 1/2 nausea and grade 1/1 vomiting; both at 700 mg q12h). The highest well-tolerated dose was 360 mg q12h (doses between 360 and 700 mg were not explored). Plasma AG-348 exposure was dose dependent, with low to moderate variability in the PK parameters of AG-348 and its metabolite AGI-8702. There was a dose-dependent decrease in 2,3-DPG and increase in ATP with the effects plateauing at 360 mg q12h. Decrease in 2,3-DPG was robust after Dose 1, while the increase in ATP occurred gradually and was strongly evident at Day 8. Change from baseline in 2,3-DPG and ATP plateaued at ~300 µg/mL (~50% decrease) and ~175 µg/mL (~50% increase), respectively. After final Day 14 dose, 2,3-DPG returned to levels similar to baseline between 72 and 120 hours. ATP levels remained elevated through 120 hours post dose (Figure). Details of all un-blinded data will be presented.
Summary
AG-348 showed a robust 2,3-DPG/ATP PD glycolytic profile at well-tolerated doses in healthy volunteers. The results support proceeding with a planned phase 2 trial in patients with PK deficiency.
Keyword(s): Clinical trial, Pharmacokinetic, Phase I, Safety
Session topic: Red cells: Novel clinical aspects
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room Strauss 1
Background
Pyruvate kinase (PK) deficiency is a rare genetic disorder of metabolism resulting in chronic hemolytic anemia, with comorbidities including iron overload and multi-organ dysfunction. Approximately 180 mutations in the PKLR gene are recognized to cause functional deficiency of the PK-R isoform. Defective glycolysis in red blood cells leads to increases in the metabolic precursor, 2,3-diphosphoglycerate (2,3-DPG), and deficiency of the product, adenosine triphosphate (ATP). AG-348 is an oral allosteric activator of both wild type and multiple mutant PK-Rs. We report here a multiple ascending dose (MAD) trial of AG-348.
Aims
To assess safety, tolerability, and pharmacokinetics/pharmacodynamics (PK/PD) of AG-348 in healthy volunteers and identify a dosing schedule for future trials in patients with PK deficiency.
Methods
A phase 1, single-center, randomized, double-blind, placebo-controlled MAD study (NCT02149966) was conducted in healthy men and women (18–60 years) in 6 sequential cohorts (each cohort: n=6 AG-348, n=2 placebo [P]). Subjects gave informed consent and received oral AG-348 at 15–700 mg twice daily (q12h) or 120 mg once daily (q24h) for 14 days with follow-up to Day 29. Adverse events (AEs), laboratory parameters, ECGs, and vital signs were monitored. Plasma AG-348 concentrations and whole blood 2,3-DPG and ATP levels were measured in serial blood samples for PK/PD assessment. Hormone levels were monitored due to pre-clinical data suggesting potential modulation.
Results
48 subjects (42M/6F), mean age 41.5 (range, 25–60) years, enrolled. Final, un-blinded safety data showed ≥1 AE in 16/36 (44%) AG-348-treated and 4/12 (33%) P subjects. Treatment-related AEs (≥1) were noted in 11/36 (31%) AG-348-treated and 3/12 (25%) P subjects. All treatment-related AEs were mild or moderate in severity (only one grade 3 event) and often reversible despite continued dosing. The most frequent AG-348-related AEs were nausea and headache, 5/36 (14%) for each (P: nausea 0/12 [0%]; headache 1/12 [8%]). Gastrointestinal AEs occurred in AG-348-treated subjects only at the highest dose, 700 mg q12h. One grade 3 AE occurred (AG-348 700 mg q12h, elevated liver function tests [LFTs], which resolved after treatment discontinuation). There were 4 AG-348 discontinuations: due to AEs in 2 subjects (grade 2 drug eruption, 60 mg q12h; grade 3 elevated LFTs, 700 mg q12h), and 2 subjects withdrew consent (both had grade 1/2 nausea and grade 1/1 vomiting; both at 700 mg q12h). The highest well-tolerated dose was 360 mg q12h (doses between 360 and 700 mg were not explored). Plasma AG-348 exposure was dose dependent, with low to moderate variability in the PK parameters of AG-348 and its metabolite AGI-8702. There was a dose-dependent decrease in 2,3-DPG and increase in ATP with the effects plateauing at 360 mg q12h. Decrease in 2,3-DPG was robust after Dose 1, while the increase in ATP occurred gradually and was strongly evident at Day 8. Change from baseline in 2,3-DPG and ATP plateaued at ~300 µg/mL (~50% decrease) and ~175 µg/mL (~50% increase), respectively. After final Day 14 dose, 2,3-DPG returned to levels similar to baseline between 72 and 120 hours. ATP levels remained elevated through 120 hours post dose (Figure). Details of all un-blinded data will be presented.
Summary
AG-348 showed a robust 2,3-DPG/ATP PD glycolytic profile at well-tolerated doses in healthy volunteers. The results support proceeding with a planned phase 2 trial in patients with PK deficiency.
Keyword(s): Clinical trial, Pharmacokinetic, Phase I, Safety

Session topic: Red cells: Novel clinical aspects
Abstract: S138
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room Strauss 1
Background
Pyruvate kinase (PK) deficiency is a rare genetic disorder of metabolism resulting in chronic hemolytic anemia, with comorbidities including iron overload and multi-organ dysfunction. Approximately 180 mutations in the PKLR gene are recognized to cause functional deficiency of the PK-R isoform. Defective glycolysis in red blood cells leads to increases in the metabolic precursor, 2,3-diphosphoglycerate (2,3-DPG), and deficiency of the product, adenosine triphosphate (ATP). AG-348 is an oral allosteric activator of both wild type and multiple mutant PK-Rs. We report here a multiple ascending dose (MAD) trial of AG-348.
Aims
To assess safety, tolerability, and pharmacokinetics/pharmacodynamics (PK/PD) of AG-348 in healthy volunteers and identify a dosing schedule for future trials in patients with PK deficiency.
Methods
A phase 1, single-center, randomized, double-blind, placebo-controlled MAD study (NCT02149966) was conducted in healthy men and women (18–60 years) in 6 sequential cohorts (each cohort: n=6 AG-348, n=2 placebo [P]). Subjects gave informed consent and received oral AG-348 at 15–700 mg twice daily (q12h) or 120 mg once daily (q24h) for 14 days with follow-up to Day 29. Adverse events (AEs), laboratory parameters, ECGs, and vital signs were monitored. Plasma AG-348 concentrations and whole blood 2,3-DPG and ATP levels were measured in serial blood samples for PK/PD assessment. Hormone levels were monitored due to pre-clinical data suggesting potential modulation.
Results
48 subjects (42M/6F), mean age 41.5 (range, 25–60) years, enrolled. Final, un-blinded safety data showed ≥1 AE in 16/36 (44%) AG-348-treated and 4/12 (33%) P subjects. Treatment-related AEs (≥1) were noted in 11/36 (31%) AG-348-treated and 3/12 (25%) P subjects. All treatment-related AEs were mild or moderate in severity (only one grade 3 event) and often reversible despite continued dosing. The most frequent AG-348-related AEs were nausea and headache, 5/36 (14%) for each (P: nausea 0/12 [0%]; headache 1/12 [8%]). Gastrointestinal AEs occurred in AG-348-treated subjects only at the highest dose, 700 mg q12h. One grade 3 AE occurred (AG-348 700 mg q12h, elevated liver function tests [LFTs], which resolved after treatment discontinuation). There were 4 AG-348 discontinuations: due to AEs in 2 subjects (grade 2 drug eruption, 60 mg q12h; grade 3 elevated LFTs, 700 mg q12h), and 2 subjects withdrew consent (both had grade 1/2 nausea and grade 1/1 vomiting; both at 700 mg q12h). The highest well-tolerated dose was 360 mg q12h (doses between 360 and 700 mg were not explored). Plasma AG-348 exposure was dose dependent, with low to moderate variability in the PK parameters of AG-348 and its metabolite AGI-8702. There was a dose-dependent decrease in 2,3-DPG and increase in ATP with the effects plateauing at 360 mg q12h. Decrease in 2,3-DPG was robust after Dose 1, while the increase in ATP occurred gradually and was strongly evident at Day 8. Change from baseline in 2,3-DPG and ATP plateaued at ~300 µg/mL (~50% decrease) and ~175 µg/mL (~50% increase), respectively. After final Day 14 dose, 2,3-DPG returned to levels similar to baseline between 72 and 120 hours. ATP levels remained elevated through 120 hours post dose (Figure). Details of all un-blinded data will be presented.
Summary
AG-348 showed a robust 2,3-DPG/ATP PD glycolytic profile at well-tolerated doses in healthy volunteers. The results support proceeding with a planned phase 2 trial in patients with PK deficiency.
Keyword(s): Clinical trial, Pharmacokinetic, Phase I, Safety

Session topic: Red cells: Novel clinical aspects
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room Strauss 1
Background
Pyruvate kinase (PK) deficiency is a rare genetic disorder of metabolism resulting in chronic hemolytic anemia, with comorbidities including iron overload and multi-organ dysfunction. Approximately 180 mutations in the PKLR gene are recognized to cause functional deficiency of the PK-R isoform. Defective glycolysis in red blood cells leads to increases in the metabolic precursor, 2,3-diphosphoglycerate (2,3-DPG), and deficiency of the product, adenosine triphosphate (ATP). AG-348 is an oral allosteric activator of both wild type and multiple mutant PK-Rs. We report here a multiple ascending dose (MAD) trial of AG-348.
Aims
To assess safety, tolerability, and pharmacokinetics/pharmacodynamics (PK/PD) of AG-348 in healthy volunteers and identify a dosing schedule for future trials in patients with PK deficiency.
Methods
A phase 1, single-center, randomized, double-blind, placebo-controlled MAD study (NCT02149966) was conducted in healthy men and women (18–60 years) in 6 sequential cohorts (each cohort: n=6 AG-348, n=2 placebo [P]). Subjects gave informed consent and received oral AG-348 at 15–700 mg twice daily (q12h) or 120 mg once daily (q24h) for 14 days with follow-up to Day 29. Adverse events (AEs), laboratory parameters, ECGs, and vital signs were monitored. Plasma AG-348 concentrations and whole blood 2,3-DPG and ATP levels were measured in serial blood samples for PK/PD assessment. Hormone levels were monitored due to pre-clinical data suggesting potential modulation.
Results
48 subjects (42M/6F), mean age 41.5 (range, 25–60) years, enrolled. Final, un-blinded safety data showed ≥1 AE in 16/36 (44%) AG-348-treated and 4/12 (33%) P subjects. Treatment-related AEs (≥1) were noted in 11/36 (31%) AG-348-treated and 3/12 (25%) P subjects. All treatment-related AEs were mild or moderate in severity (only one grade 3 event) and often reversible despite continued dosing. The most frequent AG-348-related AEs were nausea and headache, 5/36 (14%) for each (P: nausea 0/12 [0%]; headache 1/12 [8%]). Gastrointestinal AEs occurred in AG-348-treated subjects only at the highest dose, 700 mg q12h. One grade 3 AE occurred (AG-348 700 mg q12h, elevated liver function tests [LFTs], which resolved after treatment discontinuation). There were 4 AG-348 discontinuations: due to AEs in 2 subjects (grade 2 drug eruption, 60 mg q12h; grade 3 elevated LFTs, 700 mg q12h), and 2 subjects withdrew consent (both had grade 1/2 nausea and grade 1/1 vomiting; both at 700 mg q12h). The highest well-tolerated dose was 360 mg q12h (doses between 360 and 700 mg were not explored). Plasma AG-348 exposure was dose dependent, with low to moderate variability in the PK parameters of AG-348 and its metabolite AGI-8702. There was a dose-dependent decrease in 2,3-DPG and increase in ATP with the effects plateauing at 360 mg q12h. Decrease in 2,3-DPG was robust after Dose 1, while the increase in ATP occurred gradually and was strongly evident at Day 8. Change from baseline in 2,3-DPG and ATP plateaued at ~300 µg/mL (~50% decrease) and ~175 µg/mL (~50% increase), respectively. After final Day 14 dose, 2,3-DPG returned to levels similar to baseline between 72 and 120 hours. ATP levels remained elevated through 120 hours post dose (Figure). Details of all un-blinded data will be presented.
Summary
AG-348 showed a robust 2,3-DPG/ATP PD glycolytic profile at well-tolerated doses in healthy volunteers. The results support proceeding with a planned phase 2 trial in patients with PK deficiency.
Keyword(s): Clinical trial, Pharmacokinetic, Phase I, Safety

Session topic: Red cells: Novel clinical aspects
{{ help_message }}
{{filter}}


