SUBGROUP ANALYSIS BY PRIOR TREATMENT AMONG PATIENTS WITH RELAPSED OR RELAPSED AND REFRACTORY MULTIPLE MYELOMA IN THE PANORAMA 1 STUDY OF PANOBINOSTAT OR PLACEBO PLUS BORTEZOMIB AND DEXAMETHASONE
(Abstract release date: 05/21/15)
EHA Library. Einsele H. 06/12/15; 103051; S102
Disclosure(s): University Hospital Wuerzburg
Prof. Hermann Einsele
Contributions
Contributions
Abstract
Abstract: S102
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room A2+3
Background
Panobinostat (PAN), a potent pan-deacetylase inhibitor (pan-DACi), targets aberrations in multiple myeloma (MM) biology, including epigenetics and protein metabolism. In the PANORAMA 1 phase 3 clinical trial, the addition of PAN to bortezomib (BTZ) and dexamethasone (Dex; PAN-BTZ-Dex) led to a clinically relevant and statistically significant increase in median progression-free survival (PFS) of ≈ 4 months compared with placebo (Pbo) + BTZ and Dex (Pbo-BTZ-Dex) in patients (pts) with relapsed or relapsed and refractory MM.
Aims
To better characterize the efficacy and safety of PAN-BTZ-Dex within pt populations with limited treatment options by presenting a subgroup analysis of pts from the PANORAMA1 trial based on prior treatment.
Methods
The study design was described previously (San-Miguel JF, et al. Lancet Oncol. 2014;15:1195-1206). For this subanalysis, pts were analyzed for efficacy and safety based on prior treatment characteristics, including receipt of prior immunomodulatory drugs (IMiDs); BTZ + IMiDs; and BTZ + IMiDs and ≥ 2 prior lines of therapy.
Results
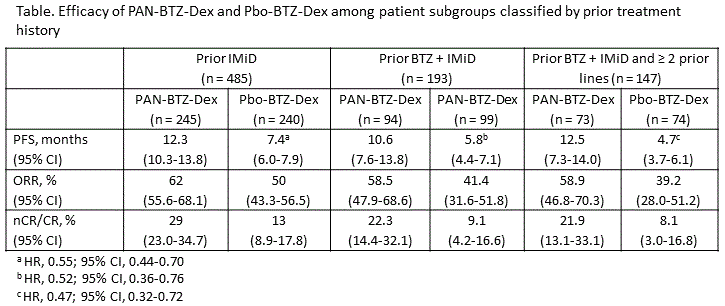
A summary of efficacy based on prior treatment is provided in the Table. Consistent or better efficacy benefit for the PAN arm was observed across all subgroups analyzed. The benefit in median PFS was particularly noteworthy among the largest subgroup in this analysis, pts who received prior IMiDs. A total of 485 pts (63%) received prior IMiDs (PAN-BTZ-Dex [n = 245] or Pbo-BTZ-Dex [n = 240]). Among these pts, the median PFS as determined by investigator assessment for the PAN arm was 12.3 months (95% CI, 10.25-13.83) vs 7.4 months (95% CI, 6.01-7.89) for the Pbo arm (hazard ratio [HR] 0.55; 95% CI, 0.44-0.70). The overall response rate for these pts was 62% (95% CI, 55.6%>68.1%) vs 50% (95% CI, 43.3%>56.5%), and ≥ near complete response (nCR) rate was 29% (95% CI, 23%>34.7%) vs 13% (95% CI, 8.9%>17.8%) in the PAN and Pbo arms, respectively. Common grade 3/4 adverse events and laboratory abnormalities in each arm included thrombocytopenia (67% vs 36%), lymphopenia (54% vs 41%), neutropenia (37% vs 13%), diarrhea (26% vs 8%), and asthenia/fatigue (25% vs 12%). The percentage of on-treatment deaths in each arm was 7.1% vs 4.2%. The safety profile was consistent among the other subgroups analyzed.
Summary
PAN-BTZ-Dex demonstrated efficacy, with consistent increases in median PFS and nCR/CR rates compared with the Pbo arm among pt subgroups with a clear unmet need, including an increase in PFS of nearly 5 months among pts who received prior IMiDs. The safety profile across these subgroups was consistent with that in the overall PANORAMA 1 population, with the exception of the frequency of on-treatment deaths, which was more similar between treatment arms across each subgroup.
Keyword(s): Bortezomib, Epigenetic, HDAC inhibitor, Myeloma
Session topic: Multiple myeloma: Clinical studies 1
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room A2+3
Background
Panobinostat (PAN), a potent pan-deacetylase inhibitor (pan-DACi), targets aberrations in multiple myeloma (MM) biology, including epigenetics and protein metabolism. In the PANORAMA 1 phase 3 clinical trial, the addition of PAN to bortezomib (BTZ) and dexamethasone (Dex; PAN-BTZ-Dex) led to a clinically relevant and statistically significant increase in median progression-free survival (PFS) of ≈ 4 months compared with placebo (Pbo) + BTZ and Dex (Pbo-BTZ-Dex) in patients (pts) with relapsed or relapsed and refractory MM.
Aims
To better characterize the efficacy and safety of PAN-BTZ-Dex within pt populations with limited treatment options by presenting a subgroup analysis of pts from the PANORAMA1 trial based on prior treatment.
Methods
The study design was described previously (San-Miguel JF, et al. Lancet Oncol. 2014;15:1195-1206). For this subanalysis, pts were analyzed for efficacy and safety based on prior treatment characteristics, including receipt of prior immunomodulatory drugs (IMiDs); BTZ + IMiDs; and BTZ + IMiDs and ≥ 2 prior lines of therapy.
Results
A summary of efficacy based on prior treatment is provided in the Table. Consistent or better efficacy benefit for the PAN arm was observed across all subgroups analyzed. The benefit in median PFS was particularly noteworthy among the largest subgroup in this analysis, pts who received prior IMiDs. A total of 485 pts (63%) received prior IMiDs (PAN-BTZ-Dex [n = 245] or Pbo-BTZ-Dex [n = 240]). Among these pts, the median PFS as determined by investigator assessment for the PAN arm was 12.3 months (95% CI, 10.25-13.83) vs 7.4 months (95% CI, 6.01-7.89) for the Pbo arm (hazard ratio [HR] 0.55; 95% CI, 0.44-0.70). The overall response rate for these pts was 62% (95% CI, 55.6%>68.1%) vs 50% (95% CI, 43.3%>56.5%), and ≥ near complete response (nCR) rate was 29% (95% CI, 23%>34.7%) vs 13% (95% CI, 8.9%>17.8%) in the PAN and Pbo arms, respectively. Common grade 3/4 adverse events and laboratory abnormalities in each arm included thrombocytopenia (67% vs 36%), lymphopenia (54% vs 41%), neutropenia (37% vs 13%), diarrhea (26% vs 8%), and asthenia/fatigue (25% vs 12%). The percentage of on-treatment deaths in each arm was 7.1% vs 4.2%. The safety profile was consistent among the other subgroups analyzed.
Summary
PAN-BTZ-Dex demonstrated efficacy, with consistent increases in median PFS and nCR/CR rates compared with the Pbo arm among pt subgroups with a clear unmet need, including an increase in PFS of nearly 5 months among pts who received prior IMiDs. The safety profile across these subgroups was consistent with that in the overall PANORAMA 1 population, with the exception of the frequency of on-treatment deaths, which was more similar between treatment arms across each subgroup.
Keyword(s): Bortezomib, Epigenetic, HDAC inhibitor, Myeloma

Session topic: Multiple myeloma: Clinical studies 1
Abstract: S102
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room A2+3
Background
Panobinostat (PAN), a potent pan-deacetylase inhibitor (pan-DACi), targets aberrations in multiple myeloma (MM) biology, including epigenetics and protein metabolism. In the PANORAMA 1 phase 3 clinical trial, the addition of PAN to bortezomib (BTZ) and dexamethasone (Dex; PAN-BTZ-Dex) led to a clinically relevant and statistically significant increase in median progression-free survival (PFS) of ≈ 4 months compared with placebo (Pbo) + BTZ and Dex (Pbo-BTZ-Dex) in patients (pts) with relapsed or relapsed and refractory MM.
Aims
To better characterize the efficacy and safety of PAN-BTZ-Dex within pt populations with limited treatment options by presenting a subgroup analysis of pts from the PANORAMA1 trial based on prior treatment.
Methods
The study design was described previously (San-Miguel JF, et al. Lancet Oncol. 2014;15:1195-1206). For this subanalysis, pts were analyzed for efficacy and safety based on prior treatment characteristics, including receipt of prior immunomodulatory drugs (IMiDs); BTZ + IMiDs; and BTZ + IMiDs and ≥ 2 prior lines of therapy.
Results
A summary of efficacy based on prior treatment is provided in the Table. Consistent or better efficacy benefit for the PAN arm was observed across all subgroups analyzed. The benefit in median PFS was particularly noteworthy among the largest subgroup in this analysis, pts who received prior IMiDs. A total of 485 pts (63%) received prior IMiDs (PAN-BTZ-Dex [n = 245] or Pbo-BTZ-Dex [n = 240]). Among these pts, the median PFS as determined by investigator assessment for the PAN arm was 12.3 months (95% CI, 10.25-13.83) vs 7.4 months (95% CI, 6.01-7.89) for the Pbo arm (hazard ratio [HR] 0.55; 95% CI, 0.44-0.70). The overall response rate for these pts was 62% (95% CI, 55.6%>68.1%) vs 50% (95% CI, 43.3%>56.5%), and ≥ near complete response (nCR) rate was 29% (95% CI, 23%>34.7%) vs 13% (95% CI, 8.9%>17.8%) in the PAN and Pbo arms, respectively. Common grade 3/4 adverse events and laboratory abnormalities in each arm included thrombocytopenia (67% vs 36%), lymphopenia (54% vs 41%), neutropenia (37% vs 13%), diarrhea (26% vs 8%), and asthenia/fatigue (25% vs 12%). The percentage of on-treatment deaths in each arm was 7.1% vs 4.2%. The safety profile was consistent among the other subgroups analyzed.
Summary
PAN-BTZ-Dex demonstrated efficacy, with consistent increases in median PFS and nCR/CR rates compared with the Pbo arm among pt subgroups with a clear unmet need, including an increase in PFS of nearly 5 months among pts who received prior IMiDs. The safety profile across these subgroups was consistent with that in the overall PANORAMA 1 population, with the exception of the frequency of on-treatment deaths, which was more similar between treatment arms across each subgroup.
Keyword(s): Bortezomib, Epigenetic, HDAC inhibitor, Myeloma

Session topic: Multiple myeloma: Clinical studies 1
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 11:45 to 12.06.2015 12:00
Location: Room A2+3
Background
Panobinostat (PAN), a potent pan-deacetylase inhibitor (pan-DACi), targets aberrations in multiple myeloma (MM) biology, including epigenetics and protein metabolism. In the PANORAMA 1 phase 3 clinical trial, the addition of PAN to bortezomib (BTZ) and dexamethasone (Dex; PAN-BTZ-Dex) led to a clinically relevant and statistically significant increase in median progression-free survival (PFS) of ≈ 4 months compared with placebo (Pbo) + BTZ and Dex (Pbo-BTZ-Dex) in patients (pts) with relapsed or relapsed and refractory MM.
Aims
To better characterize the efficacy and safety of PAN-BTZ-Dex within pt populations with limited treatment options by presenting a subgroup analysis of pts from the PANORAMA1 trial based on prior treatment.
Methods
The study design was described previously (San-Miguel JF, et al. Lancet Oncol. 2014;15:1195-1206). For this subanalysis, pts were analyzed for efficacy and safety based on prior treatment characteristics, including receipt of prior immunomodulatory drugs (IMiDs); BTZ + IMiDs; and BTZ + IMiDs and ≥ 2 prior lines of therapy.
Results
A summary of efficacy based on prior treatment is provided in the Table. Consistent or better efficacy benefit for the PAN arm was observed across all subgroups analyzed. The benefit in median PFS was particularly noteworthy among the largest subgroup in this analysis, pts who received prior IMiDs. A total of 485 pts (63%) received prior IMiDs (PAN-BTZ-Dex [n = 245] or Pbo-BTZ-Dex [n = 240]). Among these pts, the median PFS as determined by investigator assessment for the PAN arm was 12.3 months (95% CI, 10.25-13.83) vs 7.4 months (95% CI, 6.01-7.89) for the Pbo arm (hazard ratio [HR] 0.55; 95% CI, 0.44-0.70). The overall response rate for these pts was 62% (95% CI, 55.6%>68.1%) vs 50% (95% CI, 43.3%>56.5%), and ≥ near complete response (nCR) rate was 29% (95% CI, 23%>34.7%) vs 13% (95% CI, 8.9%>17.8%) in the PAN and Pbo arms, respectively. Common grade 3/4 adverse events and laboratory abnormalities in each arm included thrombocytopenia (67% vs 36%), lymphopenia (54% vs 41%), neutropenia (37% vs 13%), diarrhea (26% vs 8%), and asthenia/fatigue (25% vs 12%). The percentage of on-treatment deaths in each arm was 7.1% vs 4.2%. The safety profile was consistent among the other subgroups analyzed.
Summary
PAN-BTZ-Dex demonstrated efficacy, with consistent increases in median PFS and nCR/CR rates compared with the Pbo arm among pt subgroups with a clear unmet need, including an increase in PFS of nearly 5 months among pts who received prior IMiDs. The safety profile across these subgroups was consistent with that in the overall PANORAMA 1 population, with the exception of the frequency of on-treatment deaths, which was more similar between treatment arms across each subgroup.
Keyword(s): Bortezomib, Epigenetic, HDAC inhibitor, Myeloma

Session topic: Multiple myeloma: Clinical studies 1
{{ help_message }}
{{filter}}


