
Contributions
Type: Publication Only
Background
Tyrosin kinase inhibitors (TKI) treatment in CML has is standard of care, however, its treatment goals have become more stringent are directed by response kinetics. Since standardized BCR-ABL transcript monitoring has been established the ELN recommendations guide treatment according to the achieved molecular response. Clinical trials have demonstrated the predictive value of early molecular response with respect to consecutively achieved deep responses which in turn would allow for about half of the patient to remain in treatment free remission. In addition, the duration of sustained deep molecular response has recently been shown to be associated with a lower relapse rate after treatment discontinuation.
Although second generation TKIs (2G-TKIs) are of superior efficacy in inducing deep molecular response the majority of patients in routine care are still receiving imatinib.
Aims
Assessing the current status of treatment and response thereto is a prerequisite to ascertain a high quality of disease management and to guide physicians to future treatment options for their patients. This registry aims to reflect the real life situation of CML treatment in Austria.
Methods
The Austrian CML registry is a database project under the patronage of the ASHO approved by an ethics committee. It is focused on diagnostic parameters, e.g., conventional cytogenetics and/or FISH, BCR-ABL transcript levels, blood counts, general laboratory parameters, CML phase, concomitant diseases, CML specific treatment and including stem cell transplantation and adverse effects. Participating centres can contribute patient data after having obtained written informed consent.
Results
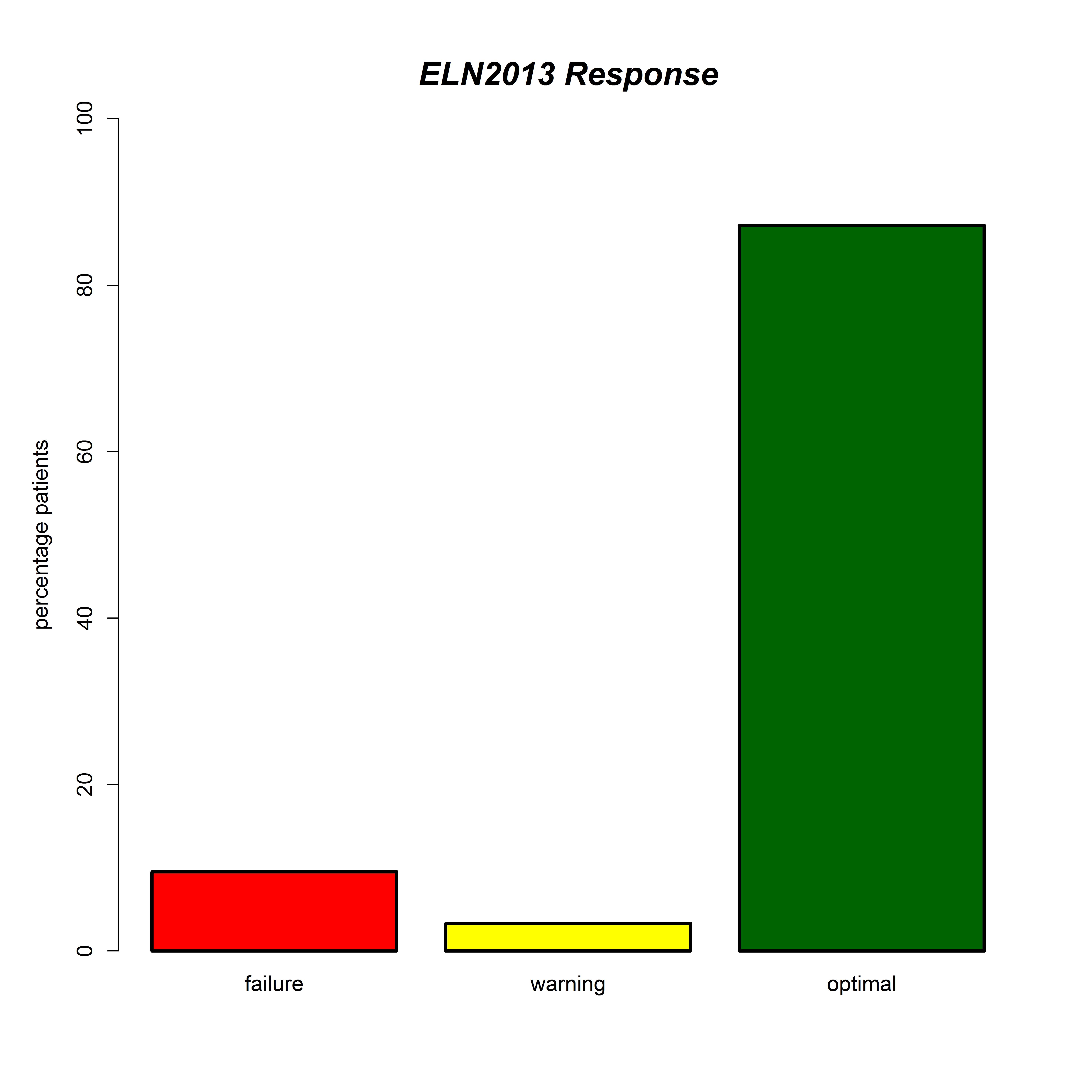
We summarize the CML registry data as of March 2015 and focus on treatment response. The CML registry cohort comprised a total of 434 patients with a median number of 4581 follow-up visits. At diagnosis 87.9%, 8.3% and 0.7% of evaluable patients were in early, late and secondary phase, respectively, whereas only 2.1%, 0.7% and 0.2% were in accelerated phase, myeloid and lymphatic blast crisis, respectively. First line treatment was imatinib, nilotinib or dasatinib in 301, 21 and 8 evaluable cases, respectively. The majority of patients had an optimal response according to ELN criteria at the last evaluable follow up (figure1). We will present the results of the currently running final update on the registry cohort.
Summary
The majority of patients fulfils the ELN 2013 criteria for optimal response. For years imatinib has been for years the mainstay of treatment and continues to be so. However, the use of 2G-TKIs has increased. The main focus of this registry for 2015 will be the percentage of patients reaching a sustained deep molecular response.
Keyword(s): Molecular response, Treatment, Tyrosine kinase inhibitor
Session topic: Publication Only
Type: Publication Only
Background
Tyrosin kinase inhibitors (TKI) treatment in CML has is standard of care, however, its treatment goals have become more stringent are directed by response kinetics. Since standardized BCR-ABL transcript monitoring has been established the ELN recommendations guide treatment according to the achieved molecular response. Clinical trials have demonstrated the predictive value of early molecular response with respect to consecutively achieved deep responses which in turn would allow for about half of the patient to remain in treatment free remission. In addition, the duration of sustained deep molecular response has recently been shown to be associated with a lower relapse rate after treatment discontinuation.
Although second generation TKIs (2G-TKIs) are of superior efficacy in inducing deep molecular response the majority of patients in routine care are still receiving imatinib.
Aims
Assessing the current status of treatment and response thereto is a prerequisite to ascertain a high quality of disease management and to guide physicians to future treatment options for their patients. This registry aims to reflect the real life situation of CML treatment in Austria.
Methods
The Austrian CML registry is a database project under the patronage of the ASHO approved by an ethics committee. It is focused on diagnostic parameters, e.g., conventional cytogenetics and/or FISH, BCR-ABL transcript levels, blood counts, general laboratory parameters, CML phase, concomitant diseases, CML specific treatment and including stem cell transplantation and adverse effects. Participating centres can contribute patient data after having obtained written informed consent.
Results
We summarize the CML registry data as of March 2015 and focus on treatment response. The CML registry cohort comprised a total of 434 patients with a median number of 4581 follow-up visits. At diagnosis 87.9%, 8.3% and 0.7% of evaluable patients were in early, late and secondary phase, respectively, whereas only 2.1%, 0.7% and 0.2% were in accelerated phase, myeloid and lymphatic blast crisis, respectively. First line treatment was imatinib, nilotinib or dasatinib in 301, 21 and 8 evaluable cases, respectively. The majority of patients had an optimal response according to ELN criteria at the last evaluable follow up (figure1). We will present the results of the currently running final update on the registry cohort.
Summary
The majority of patients fulfils the ELN 2013 criteria for optimal response. For years imatinib has been for years the mainstay of treatment and continues to be so. However, the use of 2G-TKIs has increased. The main focus of this registry for 2015 will be the percentage of patients reaching a sustained deep molecular response.
Keyword(s): Molecular response, Treatment, Tyrosine kinase inhibitor

Session topic: Publication Only


