Clinical Hematology

Contributions
Type: Publication Only
Background
Many studies have shown the prognostic value of minimal residual disease (MRD) evaluation in Acute Lymphoblastic Leukemia (ALL). Nowadays, most of the modern therapeutic protocols include risk-oriented and MRD-driven consolidation program. MRD is usually evaluated by multicolor-flow cytometry (MFC) and RQ-PCR for VDJ rearrangements. The latter method is significantly more sensible but is quite expensive and complex, and usually requires a great amount of time. MFC is cheaper and quicker, and it is currently applied in most induction protocols, where is performed at fixed timepoints.
However, few data are available on MFC MRD value outside clinical trials, where often response assessment timing greatly varies between different induction schedules and different patients.
Aims
The aim of the present study is to evaluate if MFC-MRD maintains prognostic significance in the real life therapeutic experience, outside clinical trials and with different induction schedules.
Methods
We retrospectively analyzed outcome of 132 consecutive ALL patients, treated in our centre in the last 10 years. Median age was 44.5 years (range 15-82 years). Induction regimens included Hyper-CVAD, standard three or four drug induction, with or without L-Asparaginase, and TKI with or without chemotherapy for Philadelphia positive ALL. Relapse-free survival (RFS) was calculated from the time of diagnosis until last follow-up or documented leukemic relapse. Patients were censored upon allogeneic transplantation.
MFC MRD levels were evaluated on bone marrow samples after first induction cycle, at a median of 36 days after diagnosis (range 28-52). A positive MFC MRD was defined by the presence of no less than 25 clustered leukemic cells/105 total events (threshold of 2.5x10-4 residual leukemic cells) at four-color flow-cytometry.
Results
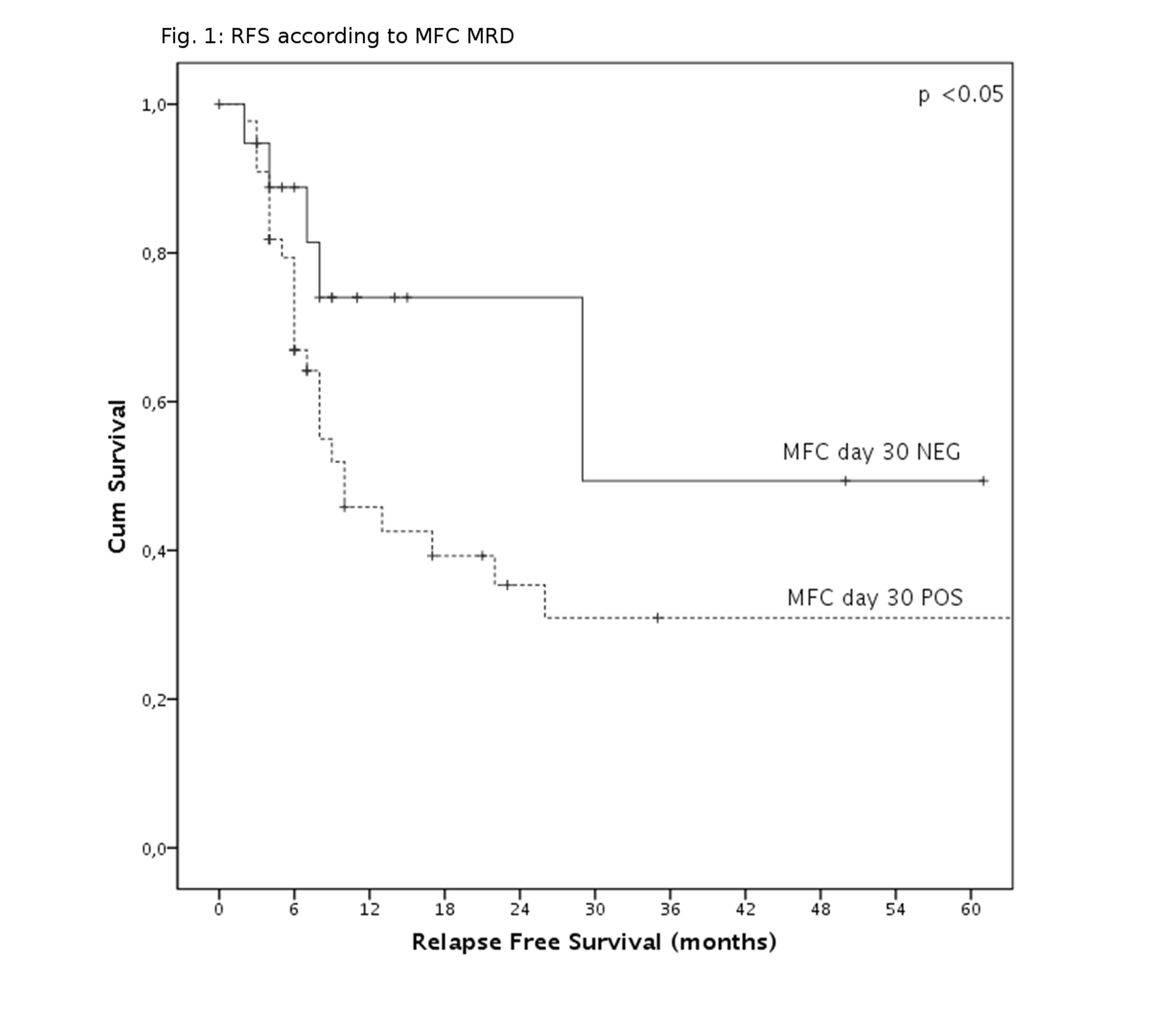
Sixteen patients (12,1%) died during induction, mainly because of infectious events. Of remaining 116 patients, 101 (84%) achieved complete remission (CR). None of the analyzed variables significantly influenced CR probability. After a median follow-up of 58 months, 63 relapses were observed. The probability of disease relapse was significantly increased by leukocytosis at diagnosis, omission of L-Asparaginase and MFC MRD positivity after first cycle (p=0.003, p=0.047, p=0.048, respectively, Fig.1). Specifically, relapse rate in MFC MRD negative patients were very low (5/25 MFC-MRD negative patients, 25%), when compared to the whole cohort. MFC value resulted independent from ALL lineage, induction schedule, sex and age. High dose Methotrexate administration conferred a borderline advantage on RFS (p=0.066).
Multivariate RFS analysis disclosed low WBC count and inclusion of L-Asparaginase as the strongest predictor of longer RFS, however without reaching statistical significance (p=0.061).
Summary
Risk adapted consolidation and transplant timing has recently shown high efficacy in many MRD-driven clinical trial. Our data confirm that evaluation of MRD by MFC on bone marrow samples is a cheap, relatively simple and a reliable predictor of relapse risk, even outside clinical trials
Basing on those findings, MFC MRD evaluation could be a valid surrogate of RQ-PCR for VDJ rearrangement, with potential clinical utility especially in those setting where access to expensive molecular techniques is difficult, allowing to apply risk adapted consolidation even if molecular MRD methodic are not available.
Keyword(s): Acute lymphoblastic leukemia, Asparaginase, Flow cytometry, Minimal residual disease (MRD)
Session topic: Publication Only
Type: Publication Only
Background
Many studies have shown the prognostic value of minimal residual disease (MRD) evaluation in Acute Lymphoblastic Leukemia (ALL). Nowadays, most of the modern therapeutic protocols include risk-oriented and MRD-driven consolidation program. MRD is usually evaluated by multicolor-flow cytometry (MFC) and RQ-PCR for VDJ rearrangements. The latter method is significantly more sensible but is quite expensive and complex, and usually requires a great amount of time. MFC is cheaper and quicker, and it is currently applied in most induction protocols, where is performed at fixed timepoints.
However, few data are available on MFC MRD value outside clinical trials, where often response assessment timing greatly varies between different induction schedules and different patients.
Aims
The aim of the present study is to evaluate if MFC-MRD maintains prognostic significance in the real life therapeutic experience, outside clinical trials and with different induction schedules.
Methods
We retrospectively analyzed outcome of 132 consecutive ALL patients, treated in our centre in the last 10 years. Median age was 44.5 years (range 15-82 years). Induction regimens included Hyper-CVAD, standard three or four drug induction, with or without L-Asparaginase, and TKI with or without chemotherapy for Philadelphia positive ALL. Relapse-free survival (RFS) was calculated from the time of diagnosis until last follow-up or documented leukemic relapse. Patients were censored upon allogeneic transplantation.
MFC MRD levels were evaluated on bone marrow samples after first induction cycle, at a median of 36 days after diagnosis (range 28-52). A positive MFC MRD was defined by the presence of no less than 25 clustered leukemic cells/105 total events (threshold of 2.5x10-4 residual leukemic cells) at four-color flow-cytometry.
Results
Sixteen patients (12,1%) died during induction, mainly because of infectious events. Of remaining 116 patients, 101 (84%) achieved complete remission (CR). None of the analyzed variables significantly influenced CR probability. After a median follow-up of 58 months, 63 relapses were observed. The probability of disease relapse was significantly increased by leukocytosis at diagnosis, omission of L-Asparaginase and MFC MRD positivity after first cycle (p=0.003, p=0.047, p=0.048, respectively, Fig.1). Specifically, relapse rate in MFC MRD negative patients were very low (5/25 MFC-MRD negative patients, 25%), when compared to the whole cohort. MFC value resulted independent from ALL lineage, induction schedule, sex and age. High dose Methotrexate administration conferred a borderline advantage on RFS (p=0.066).
Multivariate RFS analysis disclosed low WBC count and inclusion of L-Asparaginase as the strongest predictor of longer RFS, however without reaching statistical significance (p=0.061).
Summary
Risk adapted consolidation and transplant timing has recently shown high efficacy in many MRD-driven clinical trial. Our data confirm that evaluation of MRD by MFC on bone marrow samples is a cheap, relatively simple and a reliable predictor of relapse risk, even outside clinical trials
Basing on those findings, MFC MRD evaluation could be a valid surrogate of RQ-PCR for VDJ rearrangement, with potential clinical utility especially in those setting where access to expensive molecular techniques is difficult, allowing to apply risk adapted consolidation even if molecular MRD methodic are not available.
Keyword(s): Acute lymphoblastic leukemia, Asparaginase, Flow cytometry, Minimal residual disease (MRD)

Session topic: Publication Only


