HEMATOLOGY

Contributions
Type: Publication Only
Background
Hairy Cell Leukemia (HCL) is a low grade Non Hodgkin lymphoma that presents with cytopenia and splenomegaly. Diagnosis is confirmed by the presence of lymphocytes CD103, CD25, CD11c positive on bone marrow. Treatment is based of purine analogs with which patients reach an hematological response. However relapse still occurs suggesting the presence of residual disease. Recently it has been discovered the presence of B-RAF V600 mutation in HCL, and experience with B-RAF inhibitor had shown major responses. Bendamustine experience in hematologic neoplasms lead to clinical and long lasting response, especially in low grade lymphomas. The drugs acts as an alkylating agent but it shares some characteristic with purine analogs.
Aims
We previously observed its promising clinical activity with MRD eradication as well, here we report updated results.
Methods
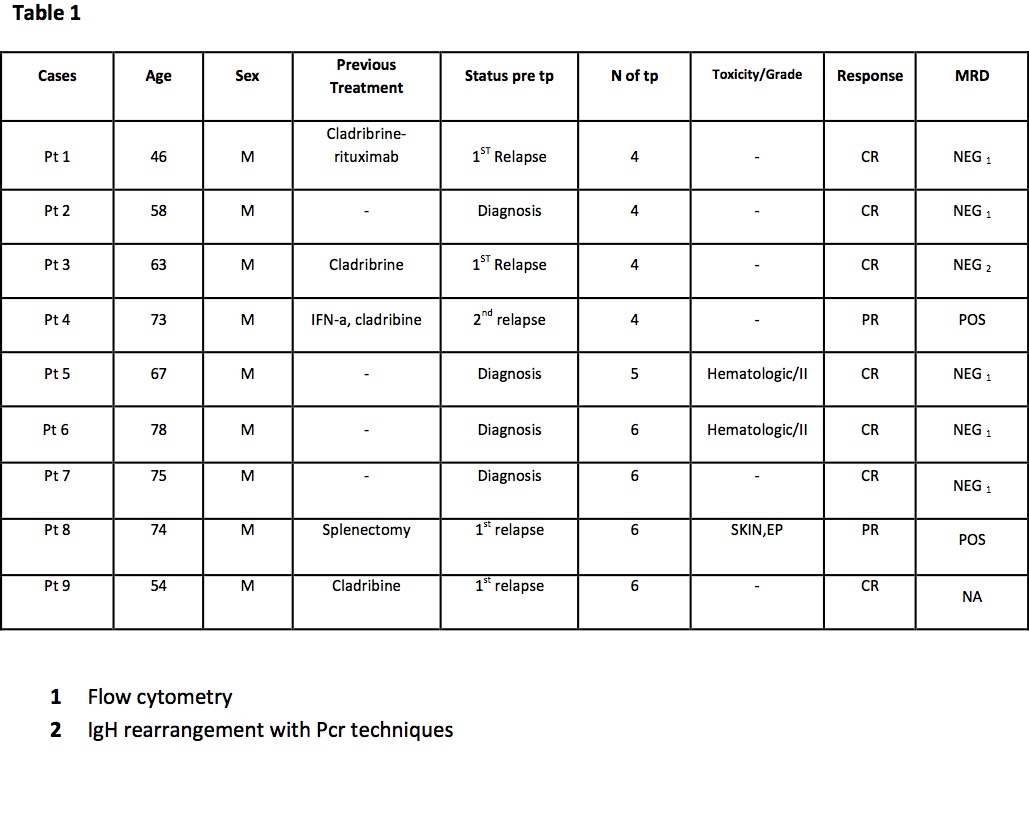
We treated a total of 9 patients (2011-2014) with bendamustine (60-70 mg/ m2 days 1,2 ) in association with rituximab (375 mg/m2 day 3). Cycles were repeated every 28 days up to 6 cycles. Before and after treatment patients had an abdomen ultrasound and bone marrow evaluation. Complete response was considered absence of marrow involvement and splenomegaly post treatment. MRD was evaluated by cytometry or IgH rearrangement with Pcr techniques. Patients’ characteristics are summarized in table 1
Results
Treatment was well tolerated in all patients, with neutropenia (grade 2) and skin toxicity (grade 2). One patient had to stop treatment due to pulmonary embolism. We did not observe any serious infective complication. Overall response rate (ORR) was 100 % with 7/9 complete response (CR) and 2/9 partial response (all in patient with relapsed disease). After a median follow up 25,7 months patients are all alive without any relapse. In evaluable patients MRD eradication has been achieved in 5/9 cases Patients treated at diagnosis obtained 100 % CR and were MRD negative
Summary
Here we confirmed our first report on bendamustine in HCL with a longer follow up and additional cases. Results are encouraging and this approach is well tolerated also in elderly patients. Data of bendamustine in HCL as first line therapy are not yet described in literature and in our experience results are at least comparable to those with purine analogs. First data on MRD are interesting as well. Future will bring B-RAF inhibitors which are not still available and with whom resistance has already been described, but in the in the meanwhile bendamustine could be suggested as a treatment option for HCL patients.
Keyword(s): Bendamustine, Hairy cell leukemia, Indolent non-Hodgkin's lymphoma, Rituximab
Session topic: Publication Only
Type: Publication Only
Background
Hairy Cell Leukemia (HCL) is a low grade Non Hodgkin lymphoma that presents with cytopenia and splenomegaly. Diagnosis is confirmed by the presence of lymphocytes CD103, CD25, CD11c positive on bone marrow. Treatment is based of purine analogs with which patients reach an hematological response. However relapse still occurs suggesting the presence of residual disease. Recently it has been discovered the presence of B-RAF V600 mutation in HCL, and experience with B-RAF inhibitor had shown major responses. Bendamustine experience in hematologic neoplasms lead to clinical and long lasting response, especially in low grade lymphomas. The drugs acts as an alkylating agent but it shares some characteristic with purine analogs.
Aims
We previously observed its promising clinical activity with MRD eradication as well, here we report updated results.
Methods
We treated a total of 9 patients (2011-2014) with bendamustine (60-70 mg/ m2 days 1,2 ) in association with rituximab (375 mg/m2 day 3). Cycles were repeated every 28 days up to 6 cycles. Before and after treatment patients had an abdomen ultrasound and bone marrow evaluation. Complete response was considered absence of marrow involvement and splenomegaly post treatment. MRD was evaluated by cytometry or IgH rearrangement with Pcr techniques. Patients’ characteristics are summarized in table 1
Results
Treatment was well tolerated in all patients, with neutropenia (grade 2) and skin toxicity (grade 2). One patient had to stop treatment due to pulmonary embolism. We did not observe any serious infective complication. Overall response rate (ORR) was 100 % with 7/9 complete response (CR) and 2/9 partial response (all in patient with relapsed disease). After a median follow up 25,7 months patients are all alive without any relapse. In evaluable patients MRD eradication has been achieved in 5/9 cases Patients treated at diagnosis obtained 100 % CR and were MRD negative
Summary
Here we confirmed our first report on bendamustine in HCL with a longer follow up and additional cases. Results are encouraging and this approach is well tolerated also in elderly patients. Data of bendamustine in HCL as first line therapy are not yet described in literature and in our experience results are at least comparable to those with purine analogs. First data on MRD are interesting as well. Future will bring B-RAF inhibitors which are not still available and with whom resistance has already been described, but in the in the meanwhile bendamustine could be suggested as a treatment option for HCL patients.
Keyword(s): Bendamustine, Hairy cell leukemia, Indolent non-Hodgkin's lymphoma, Rituximab

Session topic: Publication Only


