PATIENTS WHO DO NOT ACHIEVE STABLE UNDETECTABLE BCR-ABL1 AFTER LONG-TERM IMATINIB OBTAIN DEEP MOLECULAR RESPONSES AFTER SWITCHING TO DASATINIB
(Abstract release date: 05/21/15)
EHA Library. Takezako N. 06/12/15; 102972; PB1747
Disclosure(s): DISASTER MEDICAL CENTER OF JAPANHematology - National Hospital Organization Disaster Medical Center

Dr. Naoki Takezako
Contributions
Contributions
Abstract
Abstract: PB1747
Type: Publication Only
Background
Imatinib therapy has drastically changed the prognosis for patients with chronic myeloid leukemia (CML). Confirmed deep molecular response predicted significantly higher survival probabilities and no patient with confirmed deeper molecular response (i.e. MR4.5) has experienced progression (Hehlmann R et al. J Clin Oncol. 2014). However, even after 8 years of imatinib administration, the cumulative incidence of ≥2 years of undetectable BCR-ABL1 (Stable MR4.5) was 36.5% (Branford S et al. Blood. 2013). On the other hand, second generation tyrosine kinase inhibitor, nilotinib has been shown to be a more potent inhibitor of BCR-ABL than imatinib (Saglio G et al. N Engl J. 2010). Another study shows that patients with CML-CP with persistent minimal residual disease (MRD) on imatinib who switched to nilotinib experienced deeper confirmed molecular responses compared with those who remained on imatinib, and these responses were stable ( Hughes P, et al. Blood 2014). Dasatinib, another second generation tyrosine kinase inhibitor also induces fast and deep cytogenetic and molecular responses that translate to better outcomes (Jabbour E et al. Blood 2014). However, it is not clear that dasatinib has a potential for achieving further molecular response after switching. Here, we conducted a multicenter, prospective ‘CMR-CML’ study and observed molecular response of CML-CP patients treated with dasatinib.
Aims
The primary endpoint of our study was complete molecular response (CMR) rate by 18 months after switching to dasatinib.
Methods
For the assessment of molecular response, a quantification of BCR-ABL transcripts by RQ-PCR analysis was applied. The analysis of BCR-ABL transcripts were performed at registration, 1, 3, 6, 9, 12, 15, and 18 months after switching to dasatinib. Measurement of BCR-ABL transcripts was performed as described previously (Yoshida C et.al. Int J Clin Oncol .2013). Undetectable level of BCR-ABL transcript (<50 copies/μgRNA), which is equivalent to 4.16-log reduction, was defined to be CMR in this study. Comparisons between the qualitative variables were carried out using the χ2 test. These statistical analyses were performed with the software package Stata version 11 (Stata Corp LP, College Station, TX, USA). For all analyses, the P values were 2-tailed, and a P value less than 0.05 was considered to be significant.
Results
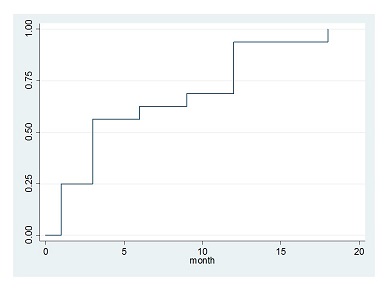
From August 6, 2011 to January 17, 2013, a total of 21 patients with CML-CP with persistent MRD after at least 2 years of imatinib therapy assigned in our study.?Five patients were excluded because of several reasons. Finally, 16 patients were registered in this trial. The median age at registration was 50 years (range, 25-70 years), and 11 were men and 5 were women. All patients received a dosage of 100mg/day dasatinib once daily. All patients achieved CMR within 18 month. There were 4, 5, 1 patients obtained CMR by 1, 3, and 6 months respectively in 16 evaluable patients (Figure). Ten patients kept CMR at 16 month and 6 patients lost CMR (2 patients widowed this trial, 3 patients was suffered from recurrence). We investigated the possible associations between the achievement of early CMR and a number of baseline variables, including age, sex, lymphocyte count, International Scale (IS), and duration of imatinib therapy. There was higher cumulative incidence of CMR at 1 month when patients with a low IS at registration were compared with those with a high IS (0.013 vs 0.034 p=0.0201). Duration of imatinib therapy was significantly longer in patients who obtained CMR in 1 month (106±21 months vs 55±10 months p=0.0322). There was no difference in the frequency of female sex, age, lymphocyte count. Dasatinib therapy was well tolerable and toxicities were almost low grade. Non-hematological toxicities were liver dysfunction (grade 1 or 2, 43%), pleural effusion (grade 1 or 2, 37%), dyspnea (grade 1, 12%; grade 3, 6%), and gastrointestinal bleeding (grade 1, 12%). Hematological toxicities were anemia (grade 1or 2, 75%; grade 3, 12%), neutropenia (grade 1 or 2, 43%; grade 3, 6%), and thrombocytopenia (grade 1 or 2, 37%; grade 3, 12%).
Summary
This pilot study shows dasatinib has the ability to obtain further deep molecular response for the patients who do not achieve stable undetectable BCR-ABL1 after long-term imatinib therapy. Based on this study, randomized control study should be attempted in the future.
Keyword(s): Chronic myeloid leukemia, Imatinib resistance
Session topic: Publication Only
Type: Publication Only
Background
Imatinib therapy has drastically changed the prognosis for patients with chronic myeloid leukemia (CML). Confirmed deep molecular response predicted significantly higher survival probabilities and no patient with confirmed deeper molecular response (i.e. MR4.5) has experienced progression (Hehlmann R et al. J Clin Oncol. 2014). However, even after 8 years of imatinib administration, the cumulative incidence of ≥2 years of undetectable BCR-ABL1 (Stable MR4.5) was 36.5% (Branford S et al. Blood. 2013). On the other hand, second generation tyrosine kinase inhibitor, nilotinib has been shown to be a more potent inhibitor of BCR-ABL than imatinib (Saglio G et al. N Engl J. 2010). Another study shows that patients with CML-CP with persistent minimal residual disease (MRD) on imatinib who switched to nilotinib experienced deeper confirmed molecular responses compared with those who remained on imatinib, and these responses were stable ( Hughes P, et al. Blood 2014). Dasatinib, another second generation tyrosine kinase inhibitor also induces fast and deep cytogenetic and molecular responses that translate to better outcomes (Jabbour E et al. Blood 2014). However, it is not clear that dasatinib has a potential for achieving further molecular response after switching. Here, we conducted a multicenter, prospective ‘CMR-CML’ study and observed molecular response of CML-CP patients treated with dasatinib.
Aims
The primary endpoint of our study was complete molecular response (CMR) rate by 18 months after switching to dasatinib.
Methods
For the assessment of molecular response, a quantification of BCR-ABL transcripts by RQ-PCR analysis was applied. The analysis of BCR-ABL transcripts were performed at registration, 1, 3, 6, 9, 12, 15, and 18 months after switching to dasatinib. Measurement of BCR-ABL transcripts was performed as described previously (Yoshida C et.al. Int J Clin Oncol .2013). Undetectable level of BCR-ABL transcript (<50 copies/μgRNA), which is equivalent to 4.16-log reduction, was defined to be CMR in this study. Comparisons between the qualitative variables were carried out using the χ2 test. These statistical analyses were performed with the software package Stata version 11 (Stata Corp LP, College Station, TX, USA). For all analyses, the P values were 2-tailed, and a P value less than 0.05 was considered to be significant.
Results
From August 6, 2011 to January 17, 2013, a total of 21 patients with CML-CP with persistent MRD after at least 2 years of imatinib therapy assigned in our study.?Five patients were excluded because of several reasons. Finally, 16 patients were registered in this trial. The median age at registration was 50 years (range, 25-70 years), and 11 were men and 5 were women. All patients received a dosage of 100mg/day dasatinib once daily. All patients achieved CMR within 18 month. There were 4, 5, 1 patients obtained CMR by 1, 3, and 6 months respectively in 16 evaluable patients (Figure). Ten patients kept CMR at 16 month and 6 patients lost CMR (2 patients widowed this trial, 3 patients was suffered from recurrence). We investigated the possible associations between the achievement of early CMR and a number of baseline variables, including age, sex, lymphocyte count, International Scale (IS), and duration of imatinib therapy. There was higher cumulative incidence of CMR at 1 month when patients with a low IS at registration were compared with those with a high IS (0.013 vs 0.034 p=0.0201). Duration of imatinib therapy was significantly longer in patients who obtained CMR in 1 month (106±21 months vs 55±10 months p=0.0322). There was no difference in the frequency of female sex, age, lymphocyte count. Dasatinib therapy was well tolerable and toxicities were almost low grade. Non-hematological toxicities were liver dysfunction (grade 1 or 2, 43%), pleural effusion (grade 1 or 2, 37%), dyspnea (grade 1, 12%; grade 3, 6%), and gastrointestinal bleeding (grade 1, 12%). Hematological toxicities were anemia (grade 1or 2, 75%; grade 3, 12%), neutropenia (grade 1 or 2, 43%; grade 3, 6%), and thrombocytopenia (grade 1 or 2, 37%; grade 3, 12%).
Summary
This pilot study shows dasatinib has the ability to obtain further deep molecular response for the patients who do not achieve stable undetectable BCR-ABL1 after long-term imatinib therapy. Based on this study, randomized control study should be attempted in the future.
Keyword(s): Chronic myeloid leukemia, Imatinib resistance

Session topic: Publication Only
Abstract: PB1747
Type: Publication Only
Background
Imatinib therapy has drastically changed the prognosis for patients with chronic myeloid leukemia (CML). Confirmed deep molecular response predicted significantly higher survival probabilities and no patient with confirmed deeper molecular response (i.e. MR4.5) has experienced progression (Hehlmann R et al. J Clin Oncol. 2014). However, even after 8 years of imatinib administration, the cumulative incidence of ≥2 years of undetectable BCR-ABL1 (Stable MR4.5) was 36.5% (Branford S et al. Blood. 2013). On the other hand, second generation tyrosine kinase inhibitor, nilotinib has been shown to be a more potent inhibitor of BCR-ABL than imatinib (Saglio G et al. N Engl J. 2010). Another study shows that patients with CML-CP with persistent minimal residual disease (MRD) on imatinib who switched to nilotinib experienced deeper confirmed molecular responses compared with those who remained on imatinib, and these responses were stable ( Hughes P, et al. Blood 2014). Dasatinib, another second generation tyrosine kinase inhibitor also induces fast and deep cytogenetic and molecular responses that translate to better outcomes (Jabbour E et al. Blood 2014). However, it is not clear that dasatinib has a potential for achieving further molecular response after switching. Here, we conducted a multicenter, prospective ‘CMR-CML’ study and observed molecular response of CML-CP patients treated with dasatinib.
Aims
The primary endpoint of our study was complete molecular response (CMR) rate by 18 months after switching to dasatinib.
Methods
For the assessment of molecular response, a quantification of BCR-ABL transcripts by RQ-PCR analysis was applied. The analysis of BCR-ABL transcripts were performed at registration, 1, 3, 6, 9, 12, 15, and 18 months after switching to dasatinib. Measurement of BCR-ABL transcripts was performed as described previously (Yoshida C et.al. Int J Clin Oncol .2013). Undetectable level of BCR-ABL transcript (<50 copies/μgRNA), which is equivalent to 4.16-log reduction, was defined to be CMR in this study. Comparisons between the qualitative variables were carried out using the χ2 test. These statistical analyses were performed with the software package Stata version 11 (Stata Corp LP, College Station, TX, USA). For all analyses, the P values were 2-tailed, and a P value less than 0.05 was considered to be significant.
Results
From August 6, 2011 to January 17, 2013, a total of 21 patients with CML-CP with persistent MRD after at least 2 years of imatinib therapy assigned in our study.?Five patients were excluded because of several reasons. Finally, 16 patients were registered in this trial. The median age at registration was 50 years (range, 25-70 years), and 11 were men and 5 were women. All patients received a dosage of 100mg/day dasatinib once daily. All patients achieved CMR within 18 month. There were 4, 5, 1 patients obtained CMR by 1, 3, and 6 months respectively in 16 evaluable patients (Figure). Ten patients kept CMR at 16 month and 6 patients lost CMR (2 patients widowed this trial, 3 patients was suffered from recurrence). We investigated the possible associations between the achievement of early CMR and a number of baseline variables, including age, sex, lymphocyte count, International Scale (IS), and duration of imatinib therapy. There was higher cumulative incidence of CMR at 1 month when patients with a low IS at registration were compared with those with a high IS (0.013 vs 0.034 p=0.0201). Duration of imatinib therapy was significantly longer in patients who obtained CMR in 1 month (106±21 months vs 55±10 months p=0.0322). There was no difference in the frequency of female sex, age, lymphocyte count. Dasatinib therapy was well tolerable and toxicities were almost low grade. Non-hematological toxicities were liver dysfunction (grade 1 or 2, 43%), pleural effusion (grade 1 or 2, 37%), dyspnea (grade 1, 12%; grade 3, 6%), and gastrointestinal bleeding (grade 1, 12%). Hematological toxicities were anemia (grade 1or 2, 75%; grade 3, 12%), neutropenia (grade 1 or 2, 43%; grade 3, 6%), and thrombocytopenia (grade 1 or 2, 37%; grade 3, 12%).
Summary
This pilot study shows dasatinib has the ability to obtain further deep molecular response for the patients who do not achieve stable undetectable BCR-ABL1 after long-term imatinib therapy. Based on this study, randomized control study should be attempted in the future.
Keyword(s): Chronic myeloid leukemia, Imatinib resistance

Session topic: Publication Only
Type: Publication Only
Background
Imatinib therapy has drastically changed the prognosis for patients with chronic myeloid leukemia (CML). Confirmed deep molecular response predicted significantly higher survival probabilities and no patient with confirmed deeper molecular response (i.e. MR4.5) has experienced progression (Hehlmann R et al. J Clin Oncol. 2014). However, even after 8 years of imatinib administration, the cumulative incidence of ≥2 years of undetectable BCR-ABL1 (Stable MR4.5) was 36.5% (Branford S et al. Blood. 2013). On the other hand, second generation tyrosine kinase inhibitor, nilotinib has been shown to be a more potent inhibitor of BCR-ABL than imatinib (Saglio G et al. N Engl J. 2010). Another study shows that patients with CML-CP with persistent minimal residual disease (MRD) on imatinib who switched to nilotinib experienced deeper confirmed molecular responses compared with those who remained on imatinib, and these responses were stable ( Hughes P, et al. Blood 2014). Dasatinib, another second generation tyrosine kinase inhibitor also induces fast and deep cytogenetic and molecular responses that translate to better outcomes (Jabbour E et al. Blood 2014). However, it is not clear that dasatinib has a potential for achieving further molecular response after switching. Here, we conducted a multicenter, prospective ‘CMR-CML’ study and observed molecular response of CML-CP patients treated with dasatinib.
Aims
The primary endpoint of our study was complete molecular response (CMR) rate by 18 months after switching to dasatinib.
Methods
For the assessment of molecular response, a quantification of BCR-ABL transcripts by RQ-PCR analysis was applied. The analysis of BCR-ABL transcripts were performed at registration, 1, 3, 6, 9, 12, 15, and 18 months after switching to dasatinib. Measurement of BCR-ABL transcripts was performed as described previously (Yoshida C et.al. Int J Clin Oncol .2013). Undetectable level of BCR-ABL transcript (<50 copies/μgRNA), which is equivalent to 4.16-log reduction, was defined to be CMR in this study. Comparisons between the qualitative variables were carried out using the χ2 test. These statistical analyses were performed with the software package Stata version 11 (Stata Corp LP, College Station, TX, USA). For all analyses, the P values were 2-tailed, and a P value less than 0.05 was considered to be significant.
Results
From August 6, 2011 to January 17, 2013, a total of 21 patients with CML-CP with persistent MRD after at least 2 years of imatinib therapy assigned in our study.?Five patients were excluded because of several reasons. Finally, 16 patients were registered in this trial. The median age at registration was 50 years (range, 25-70 years), and 11 were men and 5 were women. All patients received a dosage of 100mg/day dasatinib once daily. All patients achieved CMR within 18 month. There were 4, 5, 1 patients obtained CMR by 1, 3, and 6 months respectively in 16 evaluable patients (Figure). Ten patients kept CMR at 16 month and 6 patients lost CMR (2 patients widowed this trial, 3 patients was suffered from recurrence). We investigated the possible associations between the achievement of early CMR and a number of baseline variables, including age, sex, lymphocyte count, International Scale (IS), and duration of imatinib therapy. There was higher cumulative incidence of CMR at 1 month when patients with a low IS at registration were compared with those with a high IS (0.013 vs 0.034 p=0.0201). Duration of imatinib therapy was significantly longer in patients who obtained CMR in 1 month (106±21 months vs 55±10 months p=0.0322). There was no difference in the frequency of female sex, age, lymphocyte count. Dasatinib therapy was well tolerable and toxicities were almost low grade. Non-hematological toxicities were liver dysfunction (grade 1 or 2, 43%), pleural effusion (grade 1 or 2, 37%), dyspnea (grade 1, 12%; grade 3, 6%), and gastrointestinal bleeding (grade 1, 12%). Hematological toxicities were anemia (grade 1or 2, 75%; grade 3, 12%), neutropenia (grade 1 or 2, 43%; grade 3, 6%), and thrombocytopenia (grade 1 or 2, 37%; grade 3, 12%).
Summary
This pilot study shows dasatinib has the ability to obtain further deep molecular response for the patients who do not achieve stable undetectable BCR-ABL1 after long-term imatinib therapy. Based on this study, randomized control study should be attempted in the future.
Keyword(s): Chronic myeloid leukemia, Imatinib resistance

Session topic: Publication Only
{{ help_message }}
{{filter}}


