hematology and oncology

Contributions
Type: Publication Only
Background
Busulfan (BU) is a frontline agent in the hematopoietic stem cell transplantation (HSCT); however, severe complications including sinusoid obstructive syndrome (SOS) have been reported, particularly with oral administration. Since intravenous BU (i.v. BU) was introduced in 2006, some reports have demonstrated i.v. BU reduces hepatic complication such as SOS and treatment related mortality in adults. However, there have been few studies in pediatric populations comparing i.v. BU and oral BU (p.o. BU).
Aims
The purpose of this study was to determine the efficacy and safety of i.v. BU in children.
Methods
We retrospectively analyzed 38 cases of hematological malignancy in which HSCT was performed with myeloablative dose of BU between January 2000 and March 2014.
Results
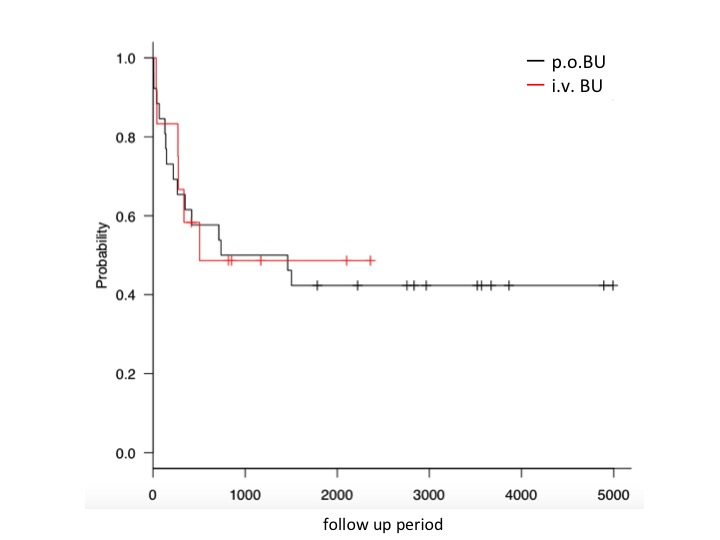
Twenty-six cases received p.o. BU and 12 cases received i.v. BU. The median age at HSCT was 5 years (range, 3months - 17 years). The median overall follow up was 777.5 days (range,7 -4893 days), 1400 days (range, 7 - 4893 days) in the p.o. BU group, and 459.5 days (range, 33 - 2360 days) in the i.v. BU group. Diagnosis of hematological malignancies were as follows: 11 AML, 10 ALL (5 infan) and 5 others in the p.o. BU group, and 10 ALL (4 infant ) and 2 others in the i.v. BU group. Disease status at HSCT with BU were as follows: 14 cases (36.8%) were CR1 overall, 11 cases (42.3%) in the p.o. BU group, and 3 cases (25%) in the i.v. BU group (P=0.32). Overall survival (OS) at 1 year was 52.5% overall, 56% in the p.o. BU group, and 50% in the i.v. BU group (P=0.83). Event free survival (EFS) was 35% overall, 42.8% in the p.o. BU group, and 33.3% in the i.v. BU group (P=0.35). Relapse rates at 1 year were 27.5% overall, 21.4% in the p.o. BU group, and 41.7% in the i.v. BU group. Non relapse mortality at 1 year was 26.3% over all, 26.9% in the p.o. BU group, and 25.0% in the i.v. BU group. SOS occurred in 6 cases (15.8%) overall, 4 (15.4%) in the p.o. BU group, and 2 (16.7 %) in the i.v. BU group (P=0.92). Adverse events above grade 3 included pulmonary complications such as interstitial pneumonia is 3.8% (2/26) in the p.o. BU group, while 50% (6/12) in the i.v. BU group. We also analyzed OS and EFS, cumulative incidence of relapse, non-relapse mortality of patiens with no prior HSCT (n=23) in this cohort. There were no statistically significant differences in OS, EFS, cumulative incidence of relapse or non-relapse mortality between p.o. BU (n=16) and i.v. BU (n=7) among patients with no prior HSCT.
Summary
In summary, our analysis demonstrated no difference in OS and EFS at 1 year or probability of SOS between myeloablative i.v. BU and p.o. BU doses for the treatment of hematological malignancies.
Recent reports have demonstrated i.v. BU can improve the outcome following HSCT in adult. However, retrospective analysis of a large number of pediatric HSCT cases in Japan found no significant benefit of i.v. BU on the survival probability (M Kato et al.Biol Blood Marrow Transplant 2013; 19: 1690-1694). Our study corroborated these result; however, this was a retrospective study with different patients backgrounds. These are the major limitations of this study. In terms of adverse events, pulmonary complication was more frequent with i.v. BU than that with p.o. BU. This result may be due to the proportion of infantile cases at a high risk of pulmonaly complications with BU included in this study.
Although i.v. BU is considered the gold - standard, i.v. BU does not confer benefit on survival probability and is still associated with severe complications including SOS and interstitial pneumonia. It is important that complications arising from the use of i.v. BU are fully elucidated by long term observational studies and the accumulation of evidence regarding the outcomes following i.v. BU use.
Session topic: Publication Only
Type: Publication Only
Background
Busulfan (BU) is a frontline agent in the hematopoietic stem cell transplantation (HSCT); however, severe complications including sinusoid obstructive syndrome (SOS) have been reported, particularly with oral administration. Since intravenous BU (i.v. BU) was introduced in 2006, some reports have demonstrated i.v. BU reduces hepatic complication such as SOS and treatment related mortality in adults. However, there have been few studies in pediatric populations comparing i.v. BU and oral BU (p.o. BU).
Aims
The purpose of this study was to determine the efficacy and safety of i.v. BU in children.
Methods
We retrospectively analyzed 38 cases of hematological malignancy in which HSCT was performed with myeloablative dose of BU between January 2000 and March 2014.
Results
Twenty-six cases received p.o. BU and 12 cases received i.v. BU. The median age at HSCT was 5 years (range, 3months - 17 years). The median overall follow up was 777.5 days (range,7 -4893 days), 1400 days (range, 7 - 4893 days) in the p.o. BU group, and 459.5 days (range, 33 - 2360 days) in the i.v. BU group. Diagnosis of hematological malignancies were as follows: 11 AML, 10 ALL (5 infan) and 5 others in the p.o. BU group, and 10 ALL (4 infant ) and 2 others in the i.v. BU group. Disease status at HSCT with BU were as follows: 14 cases (36.8%) were CR1 overall, 11 cases (42.3%) in the p.o. BU group, and 3 cases (25%) in the i.v. BU group (P=0.32). Overall survival (OS) at 1 year was 52.5% overall, 56% in the p.o. BU group, and 50% in the i.v. BU group (P=0.83). Event free survival (EFS) was 35% overall, 42.8% in the p.o. BU group, and 33.3% in the i.v. BU group (P=0.35). Relapse rates at 1 year were 27.5% overall, 21.4% in the p.o. BU group, and 41.7% in the i.v. BU group. Non relapse mortality at 1 year was 26.3% over all, 26.9% in the p.o. BU group, and 25.0% in the i.v. BU group. SOS occurred in 6 cases (15.8%) overall, 4 (15.4%) in the p.o. BU group, and 2 (16.7 %) in the i.v. BU group (P=0.92). Adverse events above grade 3 included pulmonary complications such as interstitial pneumonia is 3.8% (2/26) in the p.o. BU group, while 50% (6/12) in the i.v. BU group. We also analyzed OS and EFS, cumulative incidence of relapse, non-relapse mortality of patiens with no prior HSCT (n=23) in this cohort. There were no statistically significant differences in OS, EFS, cumulative incidence of relapse or non-relapse mortality between p.o. BU (n=16) and i.v. BU (n=7) among patients with no prior HSCT.
Summary
In summary, our analysis demonstrated no difference in OS and EFS at 1 year or probability of SOS between myeloablative i.v. BU and p.o. BU doses for the treatment of hematological malignancies.
Recent reports have demonstrated i.v. BU can improve the outcome following HSCT in adult. However, retrospective analysis of a large number of pediatric HSCT cases in Japan found no significant benefit of i.v. BU on the survival probability (M Kato et al.Biol Blood Marrow Transplant 2013; 19: 1690-1694). Our study corroborated these result; however, this was a retrospective study with different patients backgrounds. These are the major limitations of this study. In terms of adverse events, pulmonary complication was more frequent with i.v. BU than that with p.o. BU. This result may be due to the proportion of infantile cases at a high risk of pulmonaly complications with BU included in this study.
Although i.v. BU is considered the gold - standard, i.v. BU does not confer benefit on survival probability and is still associated with severe complications including SOS and interstitial pneumonia. It is important that complications arising from the use of i.v. BU are fully elucidated by long term observational studies and the accumulation of evidence regarding the outcomes following i.v. BU use.

Session topic: Publication Only


