Hematology

Contributions
Type: Publication Only
Background
With the discovery of somatic frameshift mutations in exon 9 of the calreticulin (CALR) gene, approximately 90% of myeloproliferative neoplasm (MPN) patients have gained a chance of genetic diagnosis. CALR mutations were first identified exclusively in JAK2-MPL negative essential thrombocytemia (ET) and primary myelofibrosis (PMF) at a rate of 60-88%, accounting for 1/4 to 1/3 of all patients with ET and PMF. CALR mutations that reported in the literature, consist of somatic insertions, deletions or both which always leading to an one base pair shift in the open reading frame and produce a novel C-terminal amino acid sequence that impair the function of calreticulin . As of today, more than 50 different types of mutations have been reported . The 2 most common mutations accounting for 85% of mutated cases are either a 52-bp deletion (Type 1; c.1099_1150del; L367fs*46; 44%–53% of cases) or a 5-bp insertion (Type 2; c.1154_1155insTTGTC; K385fs*47;32%–42% of cases). The remaining 15% include various other infrequent mutations that are often unique or found in a few patients.
Aims
Here we present another two CALR mutations in one patient with PMF that is not reported before.
Patient: The patient is a 46 year old man who is suffered from low back pain that have started 6 months ago. MRI scan of lumbosacral region revealed sacroileitis at the left side and he referred to a rheumatologist for further investigations. Anemia (hgb 10,8 g/dl) and thrombocytosis (700 x109/L) with high LDH level (351 U/l) were found in initial tests. The other tests for a possible rheumatologic disease including HLA-B27, were all negative and the patient was admitted to hematology clinic. Physical examination was almost normal with no sign of organomegaly. Spleen size was also normal in the abdominal ultrasound. Peripheral blood smear showed dacrocytes, occasional myelocytes (1%) and metamyelocytes (1%). Bone marrow biopsy showed diffuse, grade 3-4 reticulin fibrosis with atypical proliferation of megakaryocytes and increased cellularity consistent with PMF. BCR-ABL, JAK-2 V617F, and MPL 515L/K tests were found to be negative.
Methods
Genomic DNA was extracted from whole blood using a commercial kit (HibriGen Biotech R&D, Istanbul, Turkey). Exon 9 of the CALR gene was amplified using Forward ACAACTTCCTCATCACCAACG and Reverse GGCCTCAGTCCAGCCCTG primers. PCR reaction was performed using 2X PCR master mix (HibriGen Biotech R&D, Istanbul, Turkey), primers and 25-30 ng genomic DNA. Direct Sanger sequencing of amplified fragments were performed using BigDye Terminator Cycle Sequencing kit v3.1 and an ABI3130xl sequencer (Applied Biosystems, Foster City, CA, USA). Our results were evaluated using the BLAST (http://www.ncbi.nlm.nih.gov/BLAST) and SeqScapeTM v2.0 (Applied Biosystems, Foster City, CA, USA) software packages. Mutation was confirmed on a second DNA sample isolated from a duplicate tube of blood followed by sequencing in both forward and reverse directions. All nucleotide numbers refer to the wild type cDNA sequence of CALR (NM_004343) as reported in Ensembl.
Results
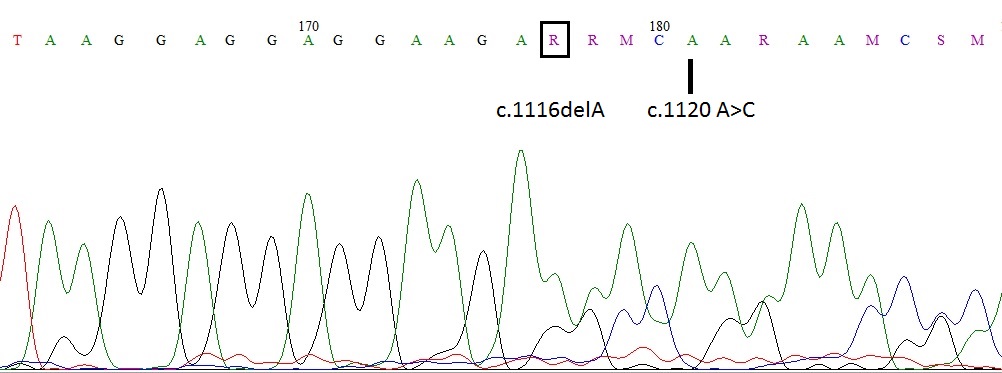
Electropherogram of CALR exon 9 of the patient was shown in figure 1. Due to a 1bp deletion in codon 372 c.1116delA (D373fs*57) and c.1120 A>C, reading frame has changed in codon 373 and there after.
Summary
To date 55 different CALR mutations have been described. Here we report two new CALR mutations [1bp deletion; c.1116delA (D373fs*57) and c.1120 A>C] in a same patient with PMF. These mutations have occured in exon 9 of the CALR gene, and changed the aminoacid sequence of C domain starting with aminoacid residue 372; which will interfere with calcium binding capacity of the molecule. The identification of new CALR mutations will improve our understanding of the pathophysiology of MPN, and will help to find new theraupetic targets.
Keyword(s): Mutation analysis, Myelofibrosis
Session topic: Publication Only
Type: Publication Only
Background
With the discovery of somatic frameshift mutations in exon 9 of the calreticulin (CALR) gene, approximately 90% of myeloproliferative neoplasm (MPN) patients have gained a chance of genetic diagnosis. CALR mutations were first identified exclusively in JAK2-MPL negative essential thrombocytemia (ET) and primary myelofibrosis (PMF) at a rate of 60-88%, accounting for 1/4 to 1/3 of all patients with ET and PMF. CALR mutations that reported in the literature, consist of somatic insertions, deletions or both which always leading to an one base pair shift in the open reading frame and produce a novel C-terminal amino acid sequence that impair the function of calreticulin . As of today, more than 50 different types of mutations have been reported . The 2 most common mutations accounting for 85% of mutated cases are either a 52-bp deletion (Type 1; c.1099_1150del; L367fs*46; 44%–53% of cases) or a 5-bp insertion (Type 2; c.1154_1155insTTGTC; K385fs*47;32%–42% of cases). The remaining 15% include various other infrequent mutations that are often unique or found in a few patients.
Aims
Here we present another two CALR mutations in one patient with PMF that is not reported before.
Patient: The patient is a 46 year old man who is suffered from low back pain that have started 6 months ago. MRI scan of lumbosacral region revealed sacroileitis at the left side and he referred to a rheumatologist for further investigations. Anemia (hgb 10,8 g/dl) and thrombocytosis (700 x109/L) with high LDH level (351 U/l) were found in initial tests. The other tests for a possible rheumatologic disease including HLA-B27, were all negative and the patient was admitted to hematology clinic. Physical examination was almost normal with no sign of organomegaly. Spleen size was also normal in the abdominal ultrasound. Peripheral blood smear showed dacrocytes, occasional myelocytes (1%) and metamyelocytes (1%). Bone marrow biopsy showed diffuse, grade 3-4 reticulin fibrosis with atypical proliferation of megakaryocytes and increased cellularity consistent with PMF. BCR-ABL, JAK-2 V617F, and MPL 515L/K tests were found to be negative.
Methods
Genomic DNA was extracted from whole blood using a commercial kit (HibriGen Biotech R&D, Istanbul, Turkey). Exon 9 of the CALR gene was amplified using Forward ACAACTTCCTCATCACCAACG and Reverse GGCCTCAGTCCAGCCCTG primers. PCR reaction was performed using 2X PCR master mix (HibriGen Biotech R&D, Istanbul, Turkey), primers and 25-30 ng genomic DNA. Direct Sanger sequencing of amplified fragments were performed using BigDye Terminator Cycle Sequencing kit v3.1 and an ABI3130xl sequencer (Applied Biosystems, Foster City, CA, USA). Our results were evaluated using the BLAST (http://www.ncbi.nlm.nih.gov/BLAST) and SeqScapeTM v2.0 (Applied Biosystems, Foster City, CA, USA) software packages. Mutation was confirmed on a second DNA sample isolated from a duplicate tube of blood followed by sequencing in both forward and reverse directions. All nucleotide numbers refer to the wild type cDNA sequence of CALR (NM_004343) as reported in Ensembl.
Results
Electropherogram of CALR exon 9 of the patient was shown in figure 1. Due to a 1bp deletion in codon 372 c.1116delA (D373fs*57) and c.1120 A>C, reading frame has changed in codon 373 and there after.
Summary
To date 55 different CALR mutations have been described. Here we report two new CALR mutations [1bp deletion; c.1116delA (D373fs*57) and c.1120 A>C] in a same patient with PMF. These mutations have occured in exon 9 of the CALR gene, and changed the aminoacid sequence of C domain starting with aminoacid residue 372; which will interfere with calcium binding capacity of the molecule. The identification of new CALR mutations will improve our understanding of the pathophysiology of MPN, and will help to find new theraupetic targets.
Keyword(s): Mutation analysis, Myelofibrosis

Session topic: Publication Only


