Pediatric Hematology/Oncology

Contributions
Type: Publication Only
Background
There is few information about nephrotoxicity than other organ damages in patients with transfusion-dependent Beta thalassemia(TDT). Creatinine levels are not sensitive indicators and do not change unless renal injury is significant. Cystatin C(Cys-C) is a marker to predict glomerular dysfunction with higher sensitivity and specificity than serum creatinine and creatinine clearance.
Aims
The aim of this study was to evaluate renal tubular and glomeruler functions with Glomerular Filtration Rate(GFR), Beta 2 microglobuline(B2 MG), mean fractional Na excretion (FENe) and cystatin C in patients with TDT.
Methods
Fifty patients with TDT who were being followed up at Istanbul University, Istanbul Medical Faculty, Thalassemia Center between January 2014 and May 2014, were included in this study after the approval of the ethical committee and informed consents were signed by patients. The mean age was 18.4±11.8 years (28 female and 22 male, age range:2-45 years). Thirty healthy volunteers with matched age and sex were also evaluated as the control group. Patients with known renal disease were excluded from our study. The patients were evaluated using GFR, FENe for glomerulopathy and serum Cys-C and urine β-2 MG for tubulopathy. These results were compared with the controls. Serum Cys-C and urine β-2 MG were measured by a latex particle-enhanced nephelometric immunoassay (DadeBehring, Liederbach, Germany).
Results
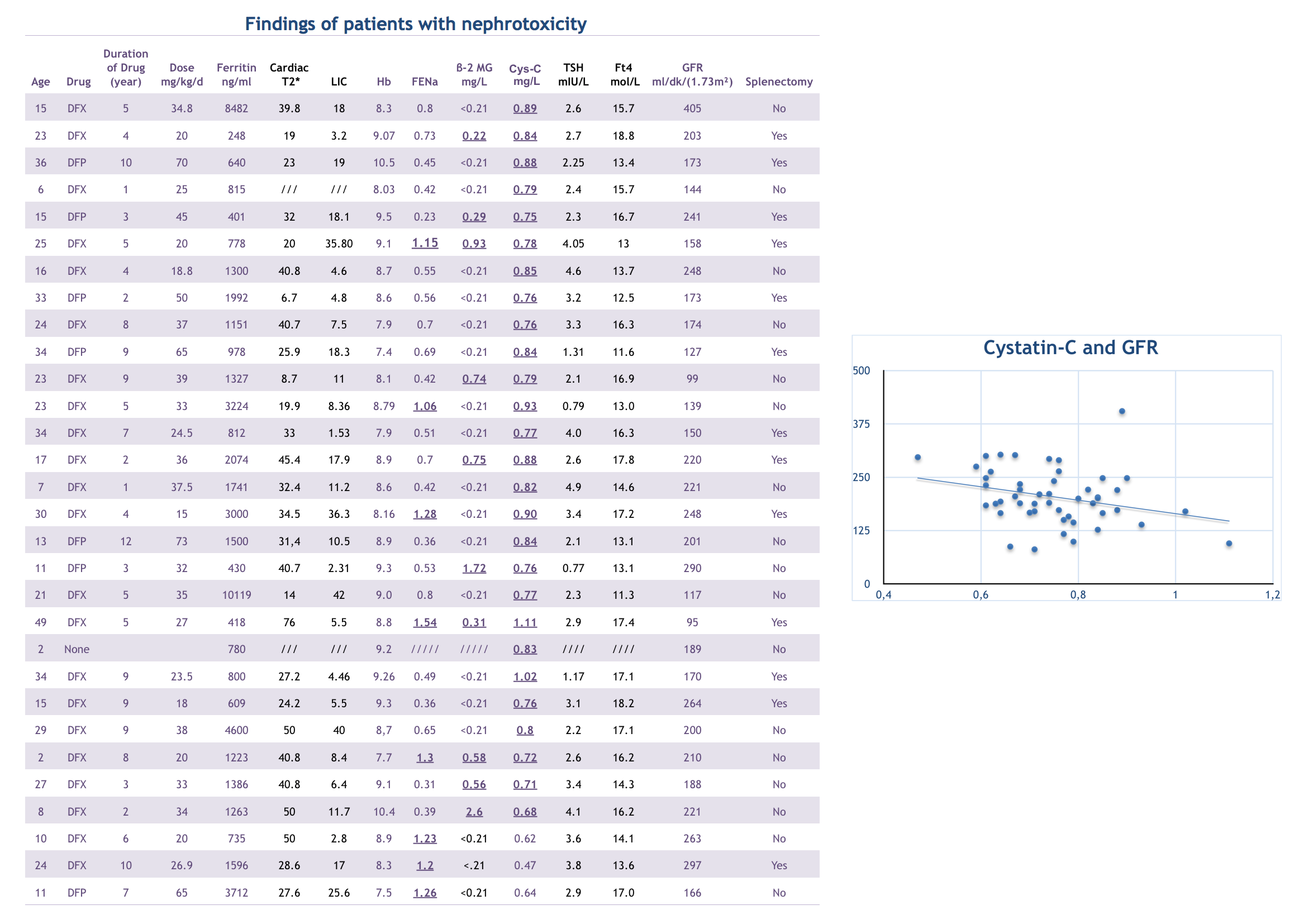
We studied 50 patients, all of them have normal GFR according to age.Thirty patients (60 %) had at least one sign of glomerulopathy and tubulopaty. Twenty-seven of 50 patients (55%) had higher serum Cys-C values (mean : 0,75+0,12) compared with controls (mean:0,66+0,09), p<0.001) while no patient had increased levels of serum Cr according to age. There was a linear relationship between Cystatin C and GFR. When we evaluated the etiology of glomerulopathy and tubulopathy, we found that; there were no statistically significant differences between pretransfusion Hb, ferritin, liver iron overload, cardiac iron overload, and cystatin C, β-2 MG, FENe and GFR. Cystatin C increases with the age, the beta-2 MG and FENe does not change with age. 27 of 30 patients with renal injury (90%) had an increased Cys-C levels compared to the control group.
Forty-two of 50 patients (84 %) used Deferasirox (DFX), 8 (16%) used Deferipron (DFP). When patients were evaluated with respect to chelation they received, nephrotoxicity was determined in 22 of 42 patients using DFX (52%). Only FENe level in 2 of these patients, only Cys-C level in 11, both FENe and Cys-C levels in 2, both B2 MG and Cys-C levels in 4, all three parameters had increased in 3 of these patients. Nephrotoxicity was detected in 7 of 8 patients (85%) using DFP. Only Cys-C level increased in 4 of these patients (57%), only FENe level increased in 1, both B2 MG and Cys-C levels had increased in 2 of these patients. Also, Cys-C level increase was detected in one TDT patient who didn’t receive any chelator yet. In patients receiving DFX, nephrotoxicity was more obvious and related with dosage.
Summary
Nephrotoxicity in thalassemia patients is reported with rate of 4-80% and is multifactorial, even if DFX receiving patients have more risk, all TDT patients should be evaluated with Cys-C for early identification of asymptomatic nephrotoxicity.
We suggest intermitten evaluations (at least every 6 months) with Cys-C in addition to routine renal function tests to diagnose and prevent major renal injury in patients with TDT.
Keyword(s): Thalassemia, Toxicity
Session topic: Publication Only
Type: Publication Only
Background
There is few information about nephrotoxicity than other organ damages in patients with transfusion-dependent Beta thalassemia(TDT). Creatinine levels are not sensitive indicators and do not change unless renal injury is significant. Cystatin C(Cys-C) is a marker to predict glomerular dysfunction with higher sensitivity and specificity than serum creatinine and creatinine clearance.
Aims
The aim of this study was to evaluate renal tubular and glomeruler functions with Glomerular Filtration Rate(GFR), Beta 2 microglobuline(B2 MG), mean fractional Na excretion (FENe) and cystatin C in patients with TDT.
Methods
Fifty patients with TDT who were being followed up at Istanbul University, Istanbul Medical Faculty, Thalassemia Center between January 2014 and May 2014, were included in this study after the approval of the ethical committee and informed consents were signed by patients. The mean age was 18.4±11.8 years (28 female and 22 male, age range:2-45 years). Thirty healthy volunteers with matched age and sex were also evaluated as the control group. Patients with known renal disease were excluded from our study. The patients were evaluated using GFR, FENe for glomerulopathy and serum Cys-C and urine β-2 MG for tubulopathy. These results were compared with the controls. Serum Cys-C and urine β-2 MG were measured by a latex particle-enhanced nephelometric immunoassay (DadeBehring, Liederbach, Germany).
Results
We studied 50 patients, all of them have normal GFR according to age.Thirty patients (60 %) had at least one sign of glomerulopathy and tubulopaty. Twenty-seven of 50 patients (55%) had higher serum Cys-C values (mean : 0,75+0,12) compared with controls (mean:0,66+0,09), p<0.001) while no patient had increased levels of serum Cr according to age. There was a linear relationship between Cystatin C and GFR. When we evaluated the etiology of glomerulopathy and tubulopathy, we found that; there were no statistically significant differences between pretransfusion Hb, ferritin, liver iron overload, cardiac iron overload, and cystatin C, β-2 MG, FENe and GFR. Cystatin C increases with the age, the beta-2 MG and FENe does not change with age. 27 of 30 patients with renal injury (90%) had an increased Cys-C levels compared to the control group.
Forty-two of 50 patients (84 %) used Deferasirox (DFX), 8 (16%) used Deferipron (DFP). When patients were evaluated with respect to chelation they received, nephrotoxicity was determined in 22 of 42 patients using DFX (52%). Only FENe level in 2 of these patients, only Cys-C level in 11, both FENe and Cys-C levels in 2, both B2 MG and Cys-C levels in 4, all three parameters had increased in 3 of these patients. Nephrotoxicity was detected in 7 of 8 patients (85%) using DFP. Only Cys-C level increased in 4 of these patients (57%), only FENe level increased in 1, both B2 MG and Cys-C levels had increased in 2 of these patients. Also, Cys-C level increase was detected in one TDT patient who didn’t receive any chelator yet. In patients receiving DFX, nephrotoxicity was more obvious and related with dosage.
Summary
Nephrotoxicity in thalassemia patients is reported with rate of 4-80% and is multifactorial, even if DFX receiving patients have more risk, all TDT patients should be evaluated with Cys-C for early identification of asymptomatic nephrotoxicity.
We suggest intermitten evaluations (at least every 6 months) with Cys-C in addition to routine renal function tests to diagnose and prevent major renal injury in patients with TDT.
Keyword(s): Thalassemia, Toxicity

Session topic: Publication Only


