Haematology

Contributions
Type: Publication Only
Background
Pomalidomide is licensed in Europe for patients with relapsed / refractory myeloma, who have received at least two prior therapies including lenalidomide and bortezomib and who have progressed on their last therapy. In the phase 3 NIMBUS study, pomalidomide and dexamethasone was associated with longer progression free (PFS, 4.0 vs 1.9 months) and overall survival (OS, 12.7 vs 8.1 months) compared to dexamethasone alone (San Miguel et al 2013).
Aims
To assess the clinical efficacy of pomalidomide in a real-world setting in several large treatment centres in the UK.
Methods
Patients had measurable disease (IMWG criteria) and received at least 1 cycle of pomalidomide with dexamethasone. Disease response was assessed as per IMWG and high risk disease defined as ISS II/III plus t(4;14) +/- del(17p) (IMWG criteria). PFS and OS were measured from start of pomalidomide therapy and estimated using Kaplan-Meier method.
Results
Seventy-nine patients were identified, of whom 62 (78.5%) were suitable for inclusion in response analyses. All patients received pomalidomide (2-4mg days 1-21) with weekly dexamethasone, 30/79 (38%) received another agent(s) [clarithromycin (23), cyclophosphamide (9), carfilzomib (1), bortezomib (1)]. Patient characteristics were as follows: median age 67 years (range 40-89); isotypes were: 43 (55%) IgG, 19 (24%) IgA, 15 (19%) LC only. Median time from diagnosis was 4.9 years (range 0.5 to 18); median prior lines of therapy was 4 (range 1 to 8). Prior therapies included lenalidomide (100%), bortezomib (98%), thalidomide (84%), and autologous stem cell transplantation (61%). Seventy-three patients (92%) were refractory to their last therapy, and 58 (73%) were refractory to both bortezomib and lenalidomide. Median follow up was 6.4 months (0.92-34.5). Median number of cycles was 4 (range 1-32), and median daily dose was 4 mg. Fifteen patients (19%) had dose reductions. In those with a GFR <45ml/min at baseline, 50% (7/14) started at a dose < 4mg.
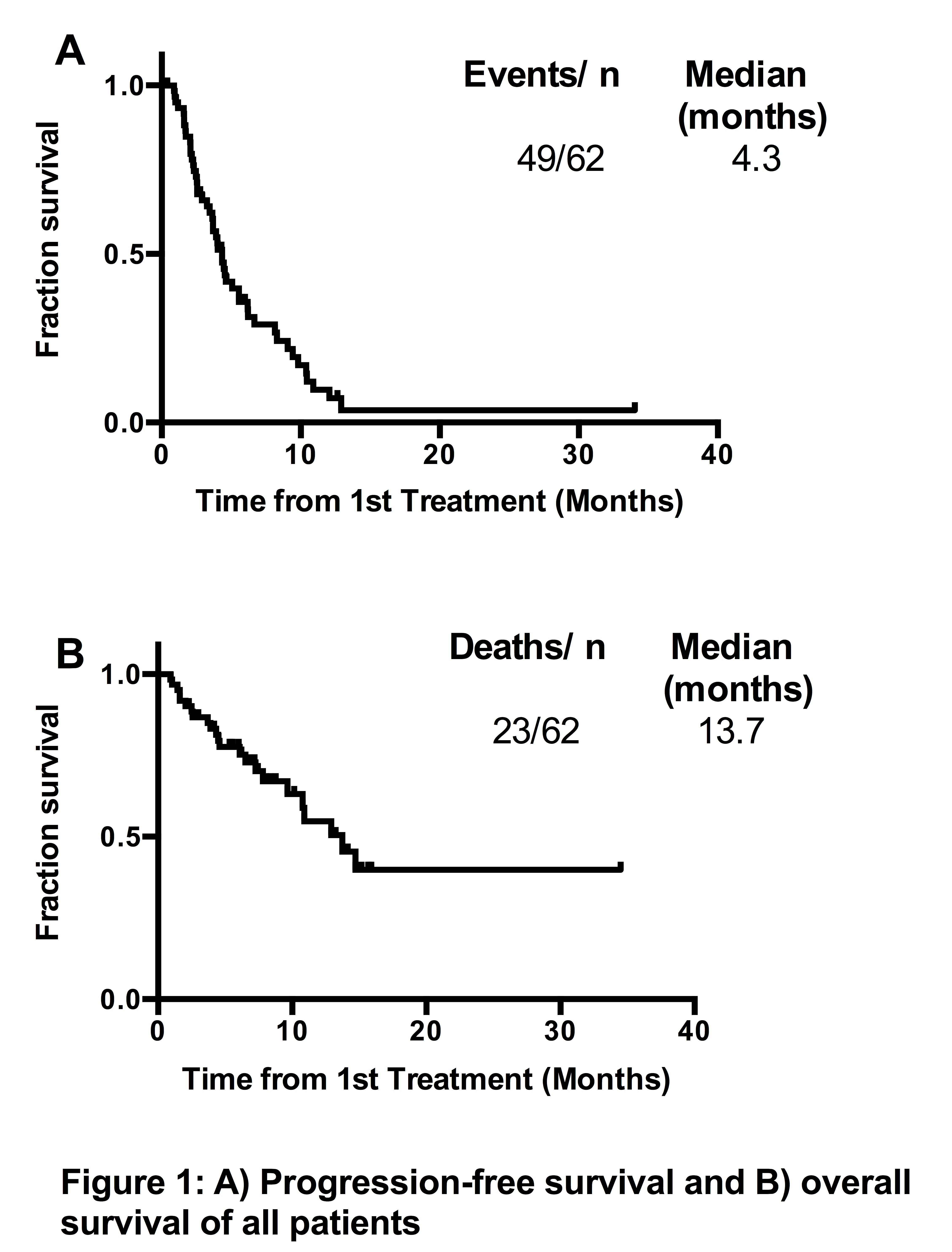
Overall response (≥ PR) was 53%, VGPR 5%, and at least stable disease was achieved in 58/62 (94%). PFS was 4.3 months (Figure 1A), and OS was 13.7 months (Figure 1B). Impaired renal function (GFR <45ml/min, 14 patients) did not appear to influence PFS (4.0 months vs 4.5 months, p=0.44), or OS (10.8 vs 13.7 months, p=0.80). High risk FISH was present in 11/40 (28%) patients, who had comparable outcomes to standard risk patients: PFS 3.6 months vs 4.5 months, (p=0.70) and OS 11.3 vs not reached (p=0.19). Inclusion of a third agent at start of therapy (14 patients) did not appear to confer benefit (PFS 4.3 vs 4.0 months, p=0.40, OS 7.8 vs 13.7 months p=0.37). In eight patients with biochemical or clinical progression on pomalidomide/dexamethasone, a third agent was added, and 7 achieved SD/PR.
Grade 3/4 non-haematological toxicities occurred in 27/79 (34%) patients: pneumonia, 15 patients (19%) and neutropenic sepsis, 9 patients (11.4%) being the most common. Grade 3/4 neutropenia occurred in 28 patients (35%) and thrombocytopenia in 17 patients (22%).
Summary
Pomalidomide is an effective treatment in relapsed / refractory myeloma, with survival outcomes comparable to reported results from the phase 3 NIMBUS study. Impaired renal function and adverse genetics do not appear to influence outcomes. The addition of a third agent should be explored prospectively.
Keyword(s): Immunomodulatory thalidomide analog, Multiple myeloma
Session topic: Publication Only
Type: Publication Only
Background
Pomalidomide is licensed in Europe for patients with relapsed / refractory myeloma, who have received at least two prior therapies including lenalidomide and bortezomib and who have progressed on their last therapy. In the phase 3 NIMBUS study, pomalidomide and dexamethasone was associated with longer progression free (PFS, 4.0 vs 1.9 months) and overall survival (OS, 12.7 vs 8.1 months) compared to dexamethasone alone (San Miguel et al 2013).
Aims
To assess the clinical efficacy of pomalidomide in a real-world setting in several large treatment centres in the UK.
Methods
Patients had measurable disease (IMWG criteria) and received at least 1 cycle of pomalidomide with dexamethasone. Disease response was assessed as per IMWG and high risk disease defined as ISS II/III plus t(4;14) +/- del(17p) (IMWG criteria). PFS and OS were measured from start of pomalidomide therapy and estimated using Kaplan-Meier method.
Results
Seventy-nine patients were identified, of whom 62 (78.5%) were suitable for inclusion in response analyses. All patients received pomalidomide (2-4mg days 1-21) with weekly dexamethasone, 30/79 (38%) received another agent(s) [clarithromycin (23), cyclophosphamide (9), carfilzomib (1), bortezomib (1)]. Patient characteristics were as follows: median age 67 years (range 40-89); isotypes were: 43 (55%) IgG, 19 (24%) IgA, 15 (19%) LC only. Median time from diagnosis was 4.9 years (range 0.5 to 18); median prior lines of therapy was 4 (range 1 to 8). Prior therapies included lenalidomide (100%), bortezomib (98%), thalidomide (84%), and autologous stem cell transplantation (61%). Seventy-three patients (92%) were refractory to their last therapy, and 58 (73%) were refractory to both bortezomib and lenalidomide. Median follow up was 6.4 months (0.92-34.5). Median number of cycles was 4 (range 1-32), and median daily dose was 4 mg. Fifteen patients (19%) had dose reductions. In those with a GFR <45ml/min at baseline, 50% (7/14) started at a dose < 4mg.
Overall response (≥ PR) was 53%, VGPR 5%, and at least stable disease was achieved in 58/62 (94%). PFS was 4.3 months (Figure 1A), and OS was 13.7 months (Figure 1B). Impaired renal function (GFR <45ml/min, 14 patients) did not appear to influence PFS (4.0 months vs 4.5 months, p=0.44), or OS (10.8 vs 13.7 months, p=0.80). High risk FISH was present in 11/40 (28%) patients, who had comparable outcomes to standard risk patients: PFS 3.6 months vs 4.5 months, (p=0.70) and OS 11.3 vs not reached (p=0.19). Inclusion of a third agent at start of therapy (14 patients) did not appear to confer benefit (PFS 4.3 vs 4.0 months, p=0.40, OS 7.8 vs 13.7 months p=0.37). In eight patients with biochemical or clinical progression on pomalidomide/dexamethasone, a third agent was added, and 7 achieved SD/PR.
Grade 3/4 non-haematological toxicities occurred in 27/79 (34%) patients: pneumonia, 15 patients (19%) and neutropenic sepsis, 9 patients (11.4%) being the most common. Grade 3/4 neutropenia occurred in 28 patients (35%) and thrombocytopenia in 17 patients (22%).
Summary
Pomalidomide is an effective treatment in relapsed / refractory myeloma, with survival outcomes comparable to reported results from the phase 3 NIMBUS study. Impaired renal function and adverse genetics do not appear to influence outcomes. The addition of a third agent should be explored prospectively.
Keyword(s): Immunomodulatory thalidomide analog, Multiple myeloma

Session topic: Publication Only


