THIRD-LINE TREATMENT WITH 2ND GENERATION TYROSINE KINASE INHIBITORS (TKIS) IN PATIENTS WITH CHRONIC MYELOID LEUKEMIA WHO FAILED TWO PRIOR TKIS: A SINGLE CENTER EXPERIENCE OF 21 PATIENTS
(Abstract release date: 05/21/15)
EHA Library. Eskazan A. 06/12/15; 102838; PB1758
Disclosure(s): Istanbul University Cerrahpasa Faculty of MedicineDepartment of Internal Medicine, Division of Hematology

Dr. Ahmet Emre Eskazan
Contributions
Contributions
Abstract
Abstract: PB1758
Type: Publication Only
Background
Although most of the chronic phase chroic myeloid leukemia (CML-CP) patients do well under imatinib (IM), some quit IM due to resistance and/or intolerance. 2nd generation tyrosine kinase inhibitors (2GTKIs) (dasatinib (DAS) and nilotinib (NIL)) can be used in patients who have intolerance and resistance to IM, and approximately 50% of patients failing to respond to previous treatments may respond to 2GTKIs. Limited data is available, displaying the outcome of CML patients who failed two lines of TKI, and received 2GTKIs as a 3rd-line treatment option.
Aims
The aim of this study is to report our single center experience on CML patients who received 2GTKIs as a 3rd-line treatment option.
Methods
Two hundred and nine CML-CP patients who were diagnosed between 1999-2013 and received IM were evaluated. Patients’ demographics, Sokal risk scores, treatment outcomes and durations, and the follow-up periods were noted from the patients’ files retrospectively. Patient monitorization was performed according to European LeukemiaNet (ELN) recommendations, and in case of resistance, mutational analysis was performed. The cumulative incidence of major molecular response (MMR) and complete cytogenetic response (CCyR) rates during the follow-up periods were calculated. The choice of 2GTKIs depended on mutational status and comorbidities of the patient, and the disease phase at the time of the switch (i.e. in patients with blastic crisis (BC), DAS was the treatment choice). Also in Turkey DAS was in the market before NIL became available, so at that period of time, DAS was the only option for 2nd-line TKI treatment.
Results
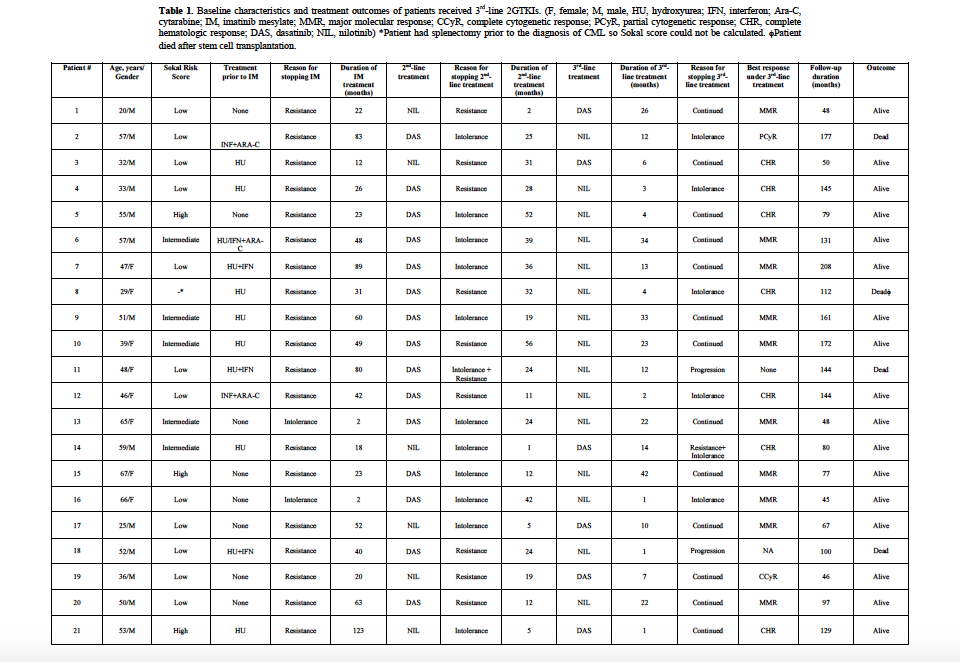
One hundred and twenty-two patients were male (58%), and the median age was 44 years (range, 18-92 years). The rates of low, intermediate and high Sokal risk scores were 44%, 39% and 17%, respectively. One hundred and seventy-eight patients (85%) were in early CP (ECP), whereas 31 (15%) were in late CP (LCP). One hundred and forty-four (68.9%) of the patients had only received IM as a TKI treatment, of which 129 (61.7%) were still on IM after a median follow-up of 86 months (range, 13-142 months) at the time of the analysis. The cumulative MMR and CCyR rates under IM were 73.6% and 79.9%, respectively. Sixty-five patients (31.1%) received 2GTKIs, of which 45 had DAS and twenty received NIL as 2nd-line treatment. One patient having an allogeneic hematopoietic stem cell transplantation (AHSCT) after IM failure received DAS for the post-transplant relapse. Among patients receiving DAS, BC was the reason for starting treatment in 5, and nine patients receiving 2nd-line DAS, and one patient receiving 2nd-line NIL died due to BC. Two patients underwent AHSCT after receiving 2nd-line DAS+chemotherapy, of which one died due to disease progression and the other one remained in remission. The cumulative MMR rates under 2nd-line DAS and NIL were 40% and 55%, respectively. Twenty-one patients (15 NIL and 6 DAS) received 2GTKIs as a 3rd-line treatment, and at the time of the analysis, 13 patients were still on treatment, five and 3 patients had to stop therapy due to intolerance and resistance, respectively. During the follow-up, four patients died (3 due to disease progression, and one after AHSCT) (Table 1). Eight patients had 3rd-line treatment for less than 6 months (median 2.5 months), and the remaining 13 had a median follow-up of 22 months under 3rd-line 2GTKIs. Ten patients achieved MMR after a median of 22.5 months (Table 1). Among patients who were refractory to 2nd-line treatment (n=10), only three had gained MMR and one achieved CCyR, whereas 7 of the remaining eleven had achieved and/or maintained MMR under 3rd-line 2GTKI treatment where switching from the 2nd-line treatment was due to intolerance.
Summary
2GTKIs can be a reasonable choice for the 3rd-line treatment even in patients who are resistant to prior 2 lines of TKI treatment, especially when 3GTKIs are not available and AHSCT is not an option.
Keyword(s): Chronic myeloid leukemia, Imatinib, Imatinib resistance, Tyrosine kinase inhibitor
Session topic: Publication Only
Type: Publication Only
Background
Although most of the chronic phase chroic myeloid leukemia (CML-CP) patients do well under imatinib (IM), some quit IM due to resistance and/or intolerance. 2nd generation tyrosine kinase inhibitors (2GTKIs) (dasatinib (DAS) and nilotinib (NIL)) can be used in patients who have intolerance and resistance to IM, and approximately 50% of patients failing to respond to previous treatments may respond to 2GTKIs. Limited data is available, displaying the outcome of CML patients who failed two lines of TKI, and received 2GTKIs as a 3rd-line treatment option.
Aims
The aim of this study is to report our single center experience on CML patients who received 2GTKIs as a 3rd-line treatment option.
Methods
Two hundred and nine CML-CP patients who were diagnosed between 1999-2013 and received IM were evaluated. Patients’ demographics, Sokal risk scores, treatment outcomes and durations, and the follow-up periods were noted from the patients’ files retrospectively. Patient monitorization was performed according to European LeukemiaNet (ELN) recommendations, and in case of resistance, mutational analysis was performed. The cumulative incidence of major molecular response (MMR) and complete cytogenetic response (CCyR) rates during the follow-up periods were calculated. The choice of 2GTKIs depended on mutational status and comorbidities of the patient, and the disease phase at the time of the switch (i.e. in patients with blastic crisis (BC), DAS was the treatment choice). Also in Turkey DAS was in the market before NIL became available, so at that period of time, DAS was the only option for 2nd-line TKI treatment.
Results
One hundred and twenty-two patients were male (58%), and the median age was 44 years (range, 18-92 years). The rates of low, intermediate and high Sokal risk scores were 44%, 39% and 17%, respectively. One hundred and seventy-eight patients (85%) were in early CP (ECP), whereas 31 (15%) were in late CP (LCP). One hundred and forty-four (68.9%) of the patients had only received IM as a TKI treatment, of which 129 (61.7%) were still on IM after a median follow-up of 86 months (range, 13-142 months) at the time of the analysis. The cumulative MMR and CCyR rates under IM were 73.6% and 79.9%, respectively. Sixty-five patients (31.1%) received 2GTKIs, of which 45 had DAS and twenty received NIL as 2nd-line treatment. One patient having an allogeneic hematopoietic stem cell transplantation (AHSCT) after IM failure received DAS for the post-transplant relapse. Among patients receiving DAS, BC was the reason for starting treatment in 5, and nine patients receiving 2nd-line DAS, and one patient receiving 2nd-line NIL died due to BC. Two patients underwent AHSCT after receiving 2nd-line DAS+chemotherapy, of which one died due to disease progression and the other one remained in remission. The cumulative MMR rates under 2nd-line DAS and NIL were 40% and 55%, respectively. Twenty-one patients (15 NIL and 6 DAS) received 2GTKIs as a 3rd-line treatment, and at the time of the analysis, 13 patients were still on treatment, five and 3 patients had to stop therapy due to intolerance and resistance, respectively. During the follow-up, four patients died (3 due to disease progression, and one after AHSCT) (Table 1). Eight patients had 3rd-line treatment for less than 6 months (median 2.5 months), and the remaining 13 had a median follow-up of 22 months under 3rd-line 2GTKIs. Ten patients achieved MMR after a median of 22.5 months (Table 1). Among patients who were refractory to 2nd-line treatment (n=10), only three had gained MMR and one achieved CCyR, whereas 7 of the remaining eleven had achieved and/or maintained MMR under 3rd-line 2GTKI treatment where switching from the 2nd-line treatment was due to intolerance.
Summary
2GTKIs can be a reasonable choice for the 3rd-line treatment even in patients who are resistant to prior 2 lines of TKI treatment, especially when 3GTKIs are not available and AHSCT is not an option.
Keyword(s): Chronic myeloid leukemia, Imatinib, Imatinib resistance, Tyrosine kinase inhibitor

Session topic: Publication Only
Abstract: PB1758
Type: Publication Only
Background
Although most of the chronic phase chroic myeloid leukemia (CML-CP) patients do well under imatinib (IM), some quit IM due to resistance and/or intolerance. 2nd generation tyrosine kinase inhibitors (2GTKIs) (dasatinib (DAS) and nilotinib (NIL)) can be used in patients who have intolerance and resistance to IM, and approximately 50% of patients failing to respond to previous treatments may respond to 2GTKIs. Limited data is available, displaying the outcome of CML patients who failed two lines of TKI, and received 2GTKIs as a 3rd-line treatment option.
Aims
The aim of this study is to report our single center experience on CML patients who received 2GTKIs as a 3rd-line treatment option.
Methods
Two hundred and nine CML-CP patients who were diagnosed between 1999-2013 and received IM were evaluated. Patients’ demographics, Sokal risk scores, treatment outcomes and durations, and the follow-up periods were noted from the patients’ files retrospectively. Patient monitorization was performed according to European LeukemiaNet (ELN) recommendations, and in case of resistance, mutational analysis was performed. The cumulative incidence of major molecular response (MMR) and complete cytogenetic response (CCyR) rates during the follow-up periods were calculated. The choice of 2GTKIs depended on mutational status and comorbidities of the patient, and the disease phase at the time of the switch (i.e. in patients with blastic crisis (BC), DAS was the treatment choice). Also in Turkey DAS was in the market before NIL became available, so at that period of time, DAS was the only option for 2nd-line TKI treatment.
Results
One hundred and twenty-two patients were male (58%), and the median age was 44 years (range, 18-92 years). The rates of low, intermediate and high Sokal risk scores were 44%, 39% and 17%, respectively. One hundred and seventy-eight patients (85%) were in early CP (ECP), whereas 31 (15%) were in late CP (LCP). One hundred and forty-four (68.9%) of the patients had only received IM as a TKI treatment, of which 129 (61.7%) were still on IM after a median follow-up of 86 months (range, 13-142 months) at the time of the analysis. The cumulative MMR and CCyR rates under IM were 73.6% and 79.9%, respectively. Sixty-five patients (31.1%) received 2GTKIs, of which 45 had DAS and twenty received NIL as 2nd-line treatment. One patient having an allogeneic hematopoietic stem cell transplantation (AHSCT) after IM failure received DAS for the post-transplant relapse. Among patients receiving DAS, BC was the reason for starting treatment in 5, and nine patients receiving 2nd-line DAS, and one patient receiving 2nd-line NIL died due to BC. Two patients underwent AHSCT after receiving 2nd-line DAS+chemotherapy, of which one died due to disease progression and the other one remained in remission. The cumulative MMR rates under 2nd-line DAS and NIL were 40% and 55%, respectively. Twenty-one patients (15 NIL and 6 DAS) received 2GTKIs as a 3rd-line treatment, and at the time of the analysis, 13 patients were still on treatment, five and 3 patients had to stop therapy due to intolerance and resistance, respectively. During the follow-up, four patients died (3 due to disease progression, and one after AHSCT) (Table 1). Eight patients had 3rd-line treatment for less than 6 months (median 2.5 months), and the remaining 13 had a median follow-up of 22 months under 3rd-line 2GTKIs. Ten patients achieved MMR after a median of 22.5 months (Table 1). Among patients who were refractory to 2nd-line treatment (n=10), only three had gained MMR and one achieved CCyR, whereas 7 of the remaining eleven had achieved and/or maintained MMR under 3rd-line 2GTKI treatment where switching from the 2nd-line treatment was due to intolerance.
Summary
2GTKIs can be a reasonable choice for the 3rd-line treatment even in patients who are resistant to prior 2 lines of TKI treatment, especially when 3GTKIs are not available and AHSCT is not an option.
Keyword(s): Chronic myeloid leukemia, Imatinib, Imatinib resistance, Tyrosine kinase inhibitor

Session topic: Publication Only
Type: Publication Only
Background
Although most of the chronic phase chroic myeloid leukemia (CML-CP) patients do well under imatinib (IM), some quit IM due to resistance and/or intolerance. 2nd generation tyrosine kinase inhibitors (2GTKIs) (dasatinib (DAS) and nilotinib (NIL)) can be used in patients who have intolerance and resistance to IM, and approximately 50% of patients failing to respond to previous treatments may respond to 2GTKIs. Limited data is available, displaying the outcome of CML patients who failed two lines of TKI, and received 2GTKIs as a 3rd-line treatment option.
Aims
The aim of this study is to report our single center experience on CML patients who received 2GTKIs as a 3rd-line treatment option.
Methods
Two hundred and nine CML-CP patients who were diagnosed between 1999-2013 and received IM were evaluated. Patients’ demographics, Sokal risk scores, treatment outcomes and durations, and the follow-up periods were noted from the patients’ files retrospectively. Patient monitorization was performed according to European LeukemiaNet (ELN) recommendations, and in case of resistance, mutational analysis was performed. The cumulative incidence of major molecular response (MMR) and complete cytogenetic response (CCyR) rates during the follow-up periods were calculated. The choice of 2GTKIs depended on mutational status and comorbidities of the patient, and the disease phase at the time of the switch (i.e. in patients with blastic crisis (BC), DAS was the treatment choice). Also in Turkey DAS was in the market before NIL became available, so at that period of time, DAS was the only option for 2nd-line TKI treatment.
Results
One hundred and twenty-two patients were male (58%), and the median age was 44 years (range, 18-92 years). The rates of low, intermediate and high Sokal risk scores were 44%, 39% and 17%, respectively. One hundred and seventy-eight patients (85%) were in early CP (ECP), whereas 31 (15%) were in late CP (LCP). One hundred and forty-four (68.9%) of the patients had only received IM as a TKI treatment, of which 129 (61.7%) were still on IM after a median follow-up of 86 months (range, 13-142 months) at the time of the analysis. The cumulative MMR and CCyR rates under IM were 73.6% and 79.9%, respectively. Sixty-five patients (31.1%) received 2GTKIs, of which 45 had DAS and twenty received NIL as 2nd-line treatment. One patient having an allogeneic hematopoietic stem cell transplantation (AHSCT) after IM failure received DAS for the post-transplant relapse. Among patients receiving DAS, BC was the reason for starting treatment in 5, and nine patients receiving 2nd-line DAS, and one patient receiving 2nd-line NIL died due to BC. Two patients underwent AHSCT after receiving 2nd-line DAS+chemotherapy, of which one died due to disease progression and the other one remained in remission. The cumulative MMR rates under 2nd-line DAS and NIL were 40% and 55%, respectively. Twenty-one patients (15 NIL and 6 DAS) received 2GTKIs as a 3rd-line treatment, and at the time of the analysis, 13 patients were still on treatment, five and 3 patients had to stop therapy due to intolerance and resistance, respectively. During the follow-up, four patients died (3 due to disease progression, and one after AHSCT) (Table 1). Eight patients had 3rd-line treatment for less than 6 months (median 2.5 months), and the remaining 13 had a median follow-up of 22 months under 3rd-line 2GTKIs. Ten patients achieved MMR after a median of 22.5 months (Table 1). Among patients who were refractory to 2nd-line treatment (n=10), only three had gained MMR and one achieved CCyR, whereas 7 of the remaining eleven had achieved and/or maintained MMR under 3rd-line 2GTKI treatment where switching from the 2nd-line treatment was due to intolerance.
Summary
2GTKIs can be a reasonable choice for the 3rd-line treatment even in patients who are resistant to prior 2 lines of TKI treatment, especially when 3GTKIs are not available and AHSCT is not an option.
Keyword(s): Chronic myeloid leukemia, Imatinib, Imatinib resistance, Tyrosine kinase inhibitor

Session topic: Publication Only
{{ help_message }}
{{filter}}


