
Contributions
Type: Publication Only
Background
In the past, acute lymphoblastic leukemia (ALL) treatment success was 20-30%. But in the last three decades this rate has been increased by over 80%. This success achieved by contemporary protocols, improvements in the infrastructures, supportive treatments, and social and psychological supports. Over the last decade, our country has reached the same success level as other developed countries. ALL BFM 95 protocol is commonly used for pediatric ALL in European countries and also in Turkey.
Aims
We would like to present our results of 147 pediatric ALL patients who were treated with this protocol in our hospital within a 10-year period.
Methods
One hundred and forty-seven children with acute lymphoblastic leukemia (ages 1-14) who were treated with ALL BFM 95 protocol in the Lösante Hospital between the years 2000 to 2009 were reviewed. The risk groups were defined by the age and leukocyte count at diagnosis, steroid response of 8th day, bone marrow aspiration results of 15th and 33th day and cytogenetics/genetic alterations.
Results
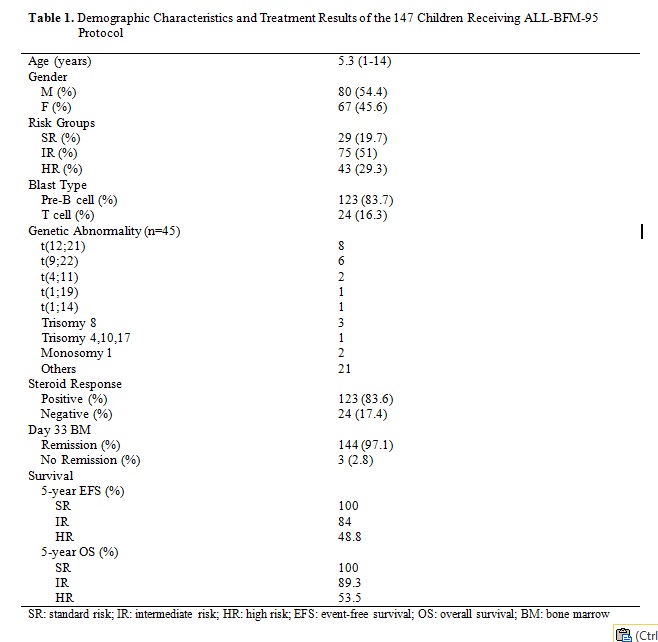
Demographic characteristics, distribution of risk groups and survival rates for children with ALL receiving ALL BFM 95 protocol are shown in Table 1. The standard risk group showed excellent results with a rate of 100% for both EFS and OS. The rates of EFS and OS for B-cell ALL are around 90% when the SR and IR groups were taken together whereas they were 55.88% and 58.82 for HR group. The event free survival (EFS) and overall survival rate (OS) for 123 patients with B-cell phenotype were 82.25% and 83.1%, respectively. The rates of OS of T-cell IR and HR groups were 86.66% and 50%, respectively. Twenty-four patients with T-cell phenotype achieved 73.91% five-year OS and 69.56% EFS rate. Overall, five-year survival rate of 147 patients who received treatment in our center was 81% and event-free survival rate was 77% that is comparable to published results so far. Regarding the white blood cell count (WBC), the rates EFS and OS are higher in patients with low WBC (<20 000/µl).
During high dose corticosteroid treatment, 8 patients showed hypertension and six patients showed hyperglycemia. Later on, patients who showed hyperglycemia diagnosed with diabetes mellitus and insulin treatment was initiated. One patient developed aseptic necrosis on femur two years after chemotherapy. PRES Syndrome was seen in two patients.
Regarding the distribution of the patients included in the risk groups, we found that number of our patients in SR group were lower (19% vs 35%), equal in IR group (51% vs 53%), and higher in HR group (30% vs 12%). This result may be due to referred patients diagnosed with ALL to our center from other medical centers. We proudly observed that the rates for EFS and OS in our ALL-BFM 95 SR group were 100%. This excellent outcome could be explained by successful planning of the current chemotherapy protocol by BFM group and implementation of appropriate supportive treatments. Additionally, our treatment results of other risk groups suggest that our success rates are comparable to the results of centers using ALL-BFM 95 protocol in European countries and our country.
Summary
We are pleased to know that ALL-BFM 95 protocol improved survival in childhood ALL. However, the development of new strategies is still necessary in order to prevent relapses and to improve survival.
Keyword(s): Acute lymphoblastic leukemia, Chemotherapy, Children
Session topic: Publication Only
Type: Publication Only
Background
In the past, acute lymphoblastic leukemia (ALL) treatment success was 20-30%. But in the last three decades this rate has been increased by over 80%. This success achieved by contemporary protocols, improvements in the infrastructures, supportive treatments, and social and psychological supports. Over the last decade, our country has reached the same success level as other developed countries. ALL BFM 95 protocol is commonly used for pediatric ALL in European countries and also in Turkey.
Aims
We would like to present our results of 147 pediatric ALL patients who were treated with this protocol in our hospital within a 10-year period.
Methods
One hundred and forty-seven children with acute lymphoblastic leukemia (ages 1-14) who were treated with ALL BFM 95 protocol in the Lösante Hospital between the years 2000 to 2009 were reviewed. The risk groups were defined by the age and leukocyte count at diagnosis, steroid response of 8th day, bone marrow aspiration results of 15th and 33th day and cytogenetics/genetic alterations.
Results
Demographic characteristics, distribution of risk groups and survival rates for children with ALL receiving ALL BFM 95 protocol are shown in Table 1. The standard risk group showed excellent results with a rate of 100% for both EFS and OS. The rates of EFS and OS for B-cell ALL are around 90% when the SR and IR groups were taken together whereas they were 55.88% and 58.82 for HR group. The event free survival (EFS) and overall survival rate (OS) for 123 patients with B-cell phenotype were 82.25% and 83.1%, respectively. The rates of OS of T-cell IR and HR groups were 86.66% and 50%, respectively. Twenty-four patients with T-cell phenotype achieved 73.91% five-year OS and 69.56% EFS rate. Overall, five-year survival rate of 147 patients who received treatment in our center was 81% and event-free survival rate was 77% that is comparable to published results so far. Regarding the white blood cell count (WBC), the rates EFS and OS are higher in patients with low WBC (<20 000/µl).
During high dose corticosteroid treatment, 8 patients showed hypertension and six patients showed hyperglycemia. Later on, patients who showed hyperglycemia diagnosed with diabetes mellitus and insulin treatment was initiated. One patient developed aseptic necrosis on femur two years after chemotherapy. PRES Syndrome was seen in two patients.
Regarding the distribution of the patients included in the risk groups, we found that number of our patients in SR group were lower (19% vs 35%), equal in IR group (51% vs 53%), and higher in HR group (30% vs 12%). This result may be due to referred patients diagnosed with ALL to our center from other medical centers. We proudly observed that the rates for EFS and OS in our ALL-BFM 95 SR group were 100%. This excellent outcome could be explained by successful planning of the current chemotherapy protocol by BFM group and implementation of appropriate supportive treatments. Additionally, our treatment results of other risk groups suggest that our success rates are comparable to the results of centers using ALL-BFM 95 protocol in European countries and our country.
Summary
We are pleased to know that ALL-BFM 95 protocol improved survival in childhood ALL. However, the development of new strategies is still necessary in order to prevent relapses and to improve survival.
Keyword(s): Acute lymphoblastic leukemia, Chemotherapy, Children

Session topic: Publication Only


