Institute of Hematology

Contributions
Type: Publication Only
Background
Estimated glomerular filtration rate (eGFR) is more accurate than serum creatinine (SCR) in assessing renal function. The IRMA study demonstrated a 50% prevalence of decreased eGFR despite normal SCR, coined as 'unrecognized renal insufficiency (RI)', among patients with solid malignancies. Unrecognized RI potentially may have an impact on treatment toxicity. In a recent study, breast cancer patients with unrecognized RI receiving cyclophosphamide and doxorubicin had an adjusted OR of 3.56 for regimen-related toxicity. However, little is known regarding its prevalence and clinical implications in hematologic malignancies.
Aims
To determine the prevalence of unrecognized RI and its association with treatment toxicity in non-Hodgkin lymphoma (NHL) patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP).
Methods
Patients ≥ 18 years with normal pre-treatment SCR (<1.3 mg/dl) and NHL treated with R-CHOP every 21 days at our institute's hemato-oncology unit (1/1/2005 – 31/8/2013) were included in this retrospective cohort. Patients who had previous chemotherapy or total bilirubin ≥ 3mg/dl were excluded. The eGFR was calculated from pre-treatment SCR, using Cockcroft-Gault (primary analysis), aMDRD and CKD-EPI equations. Joint primary outcomes were neutropenic fever and premature treatment termination, while dose reduction after cycle 1 (>20%), dose delay (≥7 days), any hospital admission and death, were secondary outcomes. Rates of treatment toxicities were compared between eGFR groups as defined by NKF-KDOQI and KDIGO guidelines, using Kendall’s Tau.
Results
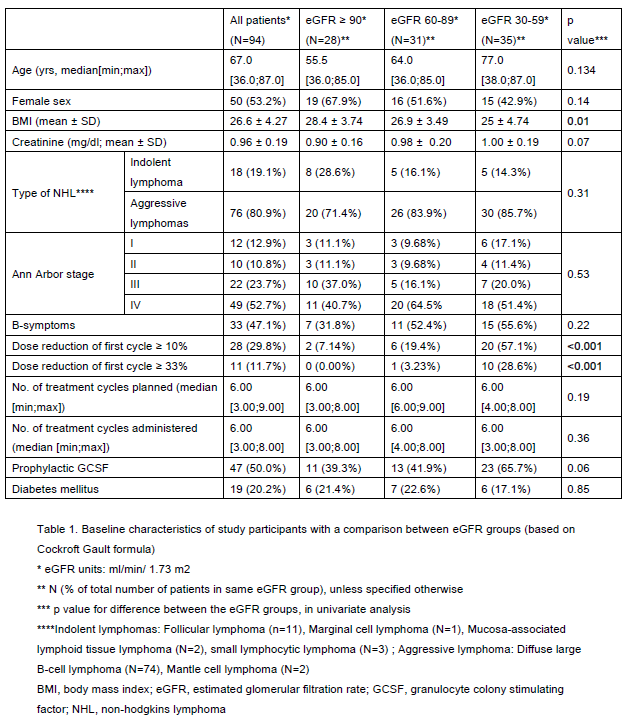
94 patients fulfilled the study criteria, reaching the planned sample size. Seventy percent had unrecognized RI distributed evenly among CKD stage 2 and 3. CHOP dose reduction ≥ 10% from cycle 1 was associated with lower eGFR groups (57% of patients with CKD3 vs 7% in CKD1), as was lower BMI. These were the only statistically significant differences in patient characteristics between CKD stages (Table 1). Patients with CKD 3 were older than patients in CKD 2 and CKD 1, although the difference was not statistically significant (p=0.134).
There was no difference in rates of neutropenic fever (overall 29.8%, p=0.68) or other regimen-related toxicities between eGFR groups. Premature treatment discontinuation was most frequent in the lowest eGFR group (p=0.03) in univariate analysis using Cockcroft Gault equation (but not with aMDRD OR EPI_CKD), but lost statistical significance after multivariate analysis. Thirty percent of patients had baseline dose reductions ≥ 10%. They were older (p<0.001) and with a lower eGFR (70% vs 22.7% CKD3, p<0.001), but had similar outcomes.
Summary
Most NHL patients treated with R-CHOP have unrecognized RI. The absence of an association between eGFR and treatment toxicity among these patients is in contrast to findings from solid malignancies. This may indicate that relatively subtle changes in renal excretion of cyclophosphamide do not carry clinical significance in this potent multidrug regimen. This could also be a false negative result, reflecting the much more common preplanned dose reductions in older patients with lower eGFR. Thus, in this population, unrecognized RI is common and had no effect on treatment toxicity when chemotherapy doses were reduced in older patients with lower eGFR, at the hematologist's discretion. Further research is needed to determine whether lower eGFR is indeed associated with treatment toxicity in a large cohort of older patients receiving full R-CHOP dose intensity, or whether it is merely a marker of older and frailer NHL patients with normal SCR.
Keyword(s): Cyclophosphamide, Lymphoma, Renal impairment
Session topic: Publication Only
Type: Publication Only
Background
Estimated glomerular filtration rate (eGFR) is more accurate than serum creatinine (SCR) in assessing renal function. The IRMA study demonstrated a 50% prevalence of decreased eGFR despite normal SCR, coined as 'unrecognized renal insufficiency (RI)', among patients with solid malignancies. Unrecognized RI potentially may have an impact on treatment toxicity. In a recent study, breast cancer patients with unrecognized RI receiving cyclophosphamide and doxorubicin had an adjusted OR of 3.56 for regimen-related toxicity. However, little is known regarding its prevalence and clinical implications in hematologic malignancies.
Aims
To determine the prevalence of unrecognized RI and its association with treatment toxicity in non-Hodgkin lymphoma (NHL) patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP).
Methods
Patients ≥ 18 years with normal pre-treatment SCR (<1.3 mg/dl) and NHL treated with R-CHOP every 21 days at our institute's hemato-oncology unit (1/1/2005 – 31/8/2013) were included in this retrospective cohort. Patients who had previous chemotherapy or total bilirubin ≥ 3mg/dl were excluded. The eGFR was calculated from pre-treatment SCR, using Cockcroft-Gault (primary analysis), aMDRD and CKD-EPI equations. Joint primary outcomes were neutropenic fever and premature treatment termination, while dose reduction after cycle 1 (>20%), dose delay (≥7 days), any hospital admission and death, were secondary outcomes. Rates of treatment toxicities were compared between eGFR groups as defined by NKF-KDOQI and KDIGO guidelines, using Kendall’s Tau.
Results
94 patients fulfilled the study criteria, reaching the planned sample size. Seventy percent had unrecognized RI distributed evenly among CKD stage 2 and 3. CHOP dose reduction ≥ 10% from cycle 1 was associated with lower eGFR groups (57% of patients with CKD3 vs 7% in CKD1), as was lower BMI. These were the only statistically significant differences in patient characteristics between CKD stages (Table 1). Patients with CKD 3 were older than patients in CKD 2 and CKD 1, although the difference was not statistically significant (p=0.134).
There was no difference in rates of neutropenic fever (overall 29.8%, p=0.68) or other regimen-related toxicities between eGFR groups. Premature treatment discontinuation was most frequent in the lowest eGFR group (p=0.03) in univariate analysis using Cockcroft Gault equation (but not with aMDRD OR EPI_CKD), but lost statistical significance after multivariate analysis. Thirty percent of patients had baseline dose reductions ≥ 10%. They were older (p<0.001) and with a lower eGFR (70% vs 22.7% CKD3, p<0.001), but had similar outcomes.
Summary
Most NHL patients treated with R-CHOP have unrecognized RI. The absence of an association between eGFR and treatment toxicity among these patients is in contrast to findings from solid malignancies. This may indicate that relatively subtle changes in renal excretion of cyclophosphamide do not carry clinical significance in this potent multidrug regimen. This could also be a false negative result, reflecting the much more common preplanned dose reductions in older patients with lower eGFR. Thus, in this population, unrecognized RI is common and had no effect on treatment toxicity when chemotherapy doses were reduced in older patients with lower eGFR, at the hematologist's discretion. Further research is needed to determine whether lower eGFR is indeed associated with treatment toxicity in a large cohort of older patients receiving full R-CHOP dose intensity, or whether it is merely a marker of older and frailer NHL patients with normal SCR.
Keyword(s): Cyclophosphamide, Lymphoma, Renal impairment

Session topic: Publication Only


