Department of Biological and Clinical Sciences

Contributions
Type: Publication Only
Background
Treatment of chronic myeloid leukemia (CML) aims at obtaining optimal response, as defined by international recommendations. As more tirosine kinase inhibitors (TKIs) are now available, much attention has been paid to the identification of early prognostic markers during treatment. A strong predictive value has been observed for early molecular response, with BCR-ABL ratio ≤ 10% at 3 months (mos), and BCR-ABL ratio ≤ 1% at 6 mos, being predictive for overall survival and event-free survival (EFS). Recently, the velocity of early BCR-ABL transcript elimination, namely the halving time, has shown to represent an additional prognostic index.
Aims
To evaluate the prognostic significance of the 3 mos time-point in our population.
Methods
We retrospectively analyzed the population of CML-CP pts treated in our Division, selecting pts who had a quantitative molecular evaluation at 3 mos. EFS was estimated with Kaplan-Meier method. Log-rank test was used to identify significant differences between curves. ROC analysis was used to calculate the optimal halving time thresholds for discriminating between outcomes. ABL was used as control gene.
Results
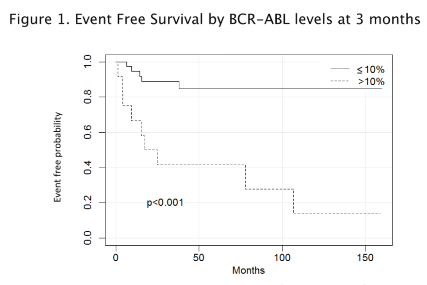
50 CML-CP pts, with a median follow-up of 57,5 mos (31-110.5) were analyzed. 36 pts (72 %) were male, 14 (28 %) female. Median age at diagnosis was 59 years. 33 pts were treated frontline with imatinib, 10 with nilotinib, 4 with dasatinib and 3 were enrolled in the GIMEMA rotational protocol nilo/ima. 76% of patients had a transcript ≤10% at 3 mos. 37 pts had the 6 mos evaluation and 39 pts had the 12 mos evaluation. 3/7 pts who had a transcript >10% at 3 mos had a transcript ≤ 1% at 6 mos (table 1). None of the patients with a transcript >10% at 3 mos achieved an MR3 at 12 mos, compared to 50% in the other group (p=0.007). 14/50 pts had an event, defined as lack or loss of optimal response as per ELN recommendations, with a statistically significant difference among groups (94.6% in the group with 3 mos BCR-ABL >10% vs 66.7% in the group with BCR-ABL ≤10%, by 12 mos, p<0.001; 84.6% in the >10% group vs 41.7% in the ≤10% group, by the median time of follow-up, p<0.001) (figure 1). No progressions occurred. 12 pts had to change drug because of an event (6 shifted to dasatinib, 6 to nilotinib), 3 for intolerance (1 shifted to dasatinib, 2 to nilotinib). Excluding these pts, in the group of pts > 10% at 3 mos the probability of achieving a long-term MR4 was more than 60% lower compared to the other group [HR 0.36 95% CI (0.10;1.27)]. The halving time thresholds for discriminating between EFS was 25.5 days (specificity 84.8% and sensitivity of 83.3%; AUC 85.9%). None of the pts with a transcript >10% at 3 mos had a halving time < 25.5 days. Among pts with a transcript ≤ 10%, those with a halving time < 25.5 days had an EFS of 93% vs 50% with a halving time > 25.5 days (p =0.012). Those with a halving time < 25.5 days had a reduction of risk of event of 92% (HR 0.08; 95% CI (0.02;035)). All pts were alive at the last follow-up.
Table 1. Responses and events according to BCR-ABL transcripts at 3 months
BCR-ABL >10% at 3 months (n=12) | BCR-ABL ≤10% at 3 months (n=38) | p-value* | ||
BCR-ABL ≤1% at 6 months | 42.9% (3/7) | 80% (24/30) | 0.07 | |
BCR-ABL ≤0.1% at 12 months | 0% (0/9) | 50% (15/30) | 0.007 | |
MR≥ 4, last follow-up | 17% (2/12) |
| 50% (19/38) | 0.05 |
Total Events | 75% (9/12) |
| 13% (5/38) | <0.001 |
*P-values were computed using Fisher’s test.
Summary
Three different drugs, imatinib, dasatinib and nilotinib. are commercially available for frontline treatment of newly diagnosed CML. Clinicians are allowed to decide which of these therapies is most suitable for each pt, based on comorbidities, Sokal risk and age of the pt. Irrespective of the TKI used, the achievement of a BCR-ABL transcript ≤ 10% influences the outcome of the pts. Interestingly, we confirmed the importance of halving time in discriminating pts with the best outcome.
Keyword(s): Chronic myeloid leukemia, Outcome, Prognostic factor
Session topic: Publication Only
Type: Publication Only
Background
Treatment of chronic myeloid leukemia (CML) aims at obtaining optimal response, as defined by international recommendations. As more tirosine kinase inhibitors (TKIs) are now available, much attention has been paid to the identification of early prognostic markers during treatment. A strong predictive value has been observed for early molecular response, with BCR-ABL ratio ≤ 10% at 3 months (mos), and BCR-ABL ratio ≤ 1% at 6 mos, being predictive for overall survival and event-free survival (EFS). Recently, the velocity of early BCR-ABL transcript elimination, namely the halving time, has shown to represent an additional prognostic index.
Aims
To evaluate the prognostic significance of the 3 mos time-point in our population.
Methods
We retrospectively analyzed the population of CML-CP pts treated in our Division, selecting pts who had a quantitative molecular evaluation at 3 mos. EFS was estimated with Kaplan-Meier method. Log-rank test was used to identify significant differences between curves. ROC analysis was used to calculate the optimal halving time thresholds for discriminating between outcomes. ABL was used as control gene.
Results
50 CML-CP pts, with a median follow-up of 57,5 mos (31-110.5) were analyzed. 36 pts (72 %) were male, 14 (28 %) female. Median age at diagnosis was 59 years. 33 pts were treated frontline with imatinib, 10 with nilotinib, 4 with dasatinib and 3 were enrolled in the GIMEMA rotational protocol nilo/ima. 76% of patients had a transcript ≤10% at 3 mos. 37 pts had the 6 mos evaluation and 39 pts had the 12 mos evaluation. 3/7 pts who had a transcript >10% at 3 mos had a transcript ≤ 1% at 6 mos (table 1). None of the patients with a transcript >10% at 3 mos achieved an MR3 at 12 mos, compared to 50% in the other group (p=0.007). 14/50 pts had an event, defined as lack or loss of optimal response as per ELN recommendations, with a statistically significant difference among groups (94.6% in the group with 3 mos BCR-ABL >10% vs 66.7% in the group with BCR-ABL ≤10%, by 12 mos, p<0.001; 84.6% in the >10% group vs 41.7% in the ≤10% group, by the median time of follow-up, p<0.001) (figure 1). No progressions occurred. 12 pts had to change drug because of an event (6 shifted to dasatinib, 6 to nilotinib), 3 for intolerance (1 shifted to dasatinib, 2 to nilotinib). Excluding these pts, in the group of pts > 10% at 3 mos the probability of achieving a long-term MR4 was more than 60% lower compared to the other group [HR 0.36 95% CI (0.10;1.27)]. The halving time thresholds for discriminating between EFS was 25.5 days (specificity 84.8% and sensitivity of 83.3%; AUC 85.9%). None of the pts with a transcript >10% at 3 mos had a halving time < 25.5 days. Among pts with a transcript ≤ 10%, those with a halving time < 25.5 days had an EFS of 93% vs 50% with a halving time > 25.5 days (p =0.012). Those with a halving time < 25.5 days had a reduction of risk of event of 92% (HR 0.08; 95% CI (0.02;035)). All pts were alive at the last follow-up.
Table 1. Responses and events according to BCR-ABL transcripts at 3 months
BCR-ABL >10% at 3 months (n=12) | BCR-ABL ≤10% at 3 months (n=38) | p-value* | ||
BCR-ABL ≤1% at 6 months | 42.9% (3/7) | 80% (24/30) | 0.07 | |
BCR-ABL ≤0.1% at 12 months | 0% (0/9) | 50% (15/30) | 0.007 | |
MR≥ 4, last follow-up | 17% (2/12) |
| 50% (19/38) | 0.05 |
Total Events | 75% (9/12) |
| 13% (5/38) | <0.001 |
*P-values were computed using Fisher’s test.
Summary
Three different drugs, imatinib, dasatinib and nilotinib. are commercially available for frontline treatment of newly diagnosed CML. Clinicians are allowed to decide which of these therapies is most suitable for each pt, based on comorbidities, Sokal risk and age of the pt. Irrespective of the TKI used, the achievement of a BCR-ABL transcript ≤ 10% influences the outcome of the pts. Interestingly, we confirmed the importance of halving time in discriminating pts with the best outcome.
Keyword(s): Chronic myeloid leukemia, Outcome, Prognostic factor

Session topic: Publication Only


