BENDAMUSTINE-BASED THERAPY RELAPSE/REFRACTORY MULTIPLE MYELOMA PATIENTS: FIRST IMPRESSIONS IN RUSSIA
(Abstract release date: 05/21/15)
EHA Library. Garifullin A. 06/12/15; 102803; PB1895
Disclosure(s): Russian Research Institute of Hematology and Transfusiology
Andrei Garifullin
Contributions
Contributions
Abstract
Abstract: PB1895
Type: Publication Only
Background
Relapsed and refractory (R/R) multiple myeloma (MM) constitutes a specific and unmet medical need. Median survival ranges from as little as 6 to 9 months, and responses to treatment are characteristically short. In patients with R/R MM after therapy of bortezomib and/or immunomodulators (IMiDs) a bendamustine-based treatment can be used as “salvage”.
Aims
Examine efficiency of bendamustine-based chemotherapy in R/R MM patients.
Methods
In this retrospective analysis we have identified 32 patients with R/R MM by means of case research, who have been bendamustine-based treated at Hematological Clinics of Russian Federation since 2011. Median age was 67 years, the female/male ratio was 2.5:1. After in median 2 lines of prior therapy (range: 1-7) patients received in median 3 (range: 1-9) cycles of bendamustine-based therapy. Bendamustine dosage was 70-120 mg/m2/day on days 1 and 2 of a each 28-day cycle until progressive disease or intolerability. Bendamustine was administered as monotherapy in 12% of patients, whereas 88% received concomitant steroids (group Bendamustine (B), n=17). Bendamustine with dexamethasone and bortezomib or IMiDs (BBD, BRD, BTD) was evaluated in 15 patients with R/R MM (group Bendamustine-plus (B+)). Primary end point was overall response rate (ORR). Secondary end points were time to progression (TTP), overall survival (OS), and toxicity.
Results
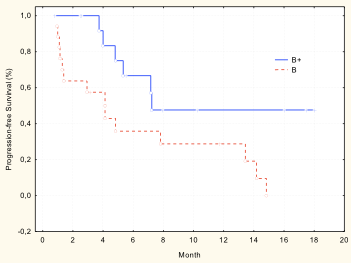
ORR in both group was 59.2%: 22.2% partial response (6.7% in group B vs. 41.7% in group B+, ?<0.05), stable disease 37.0% (26.7% vs. 50.0%, accordingly, ?>0.05). Median TTP was 9.7 months (4.1 mo in group B vs. 7.2 mo in group B+, ?=0.02)) (pic). Median OS was 25.4 months in group B, but in group B+ median OS is not reached. Hematologic toxicity was in 25.0% of patients: grade 3/4 anemia was noted in 9.4%, grade 3/4 thrombocytopenia was noted in 6.3%, and grade 3/4 infections were noted in 21.9%.
Summary
Bendamustine-based therapy is active and well tolerated in patients with relapsed/refractory multiple myeloma.
Keyword(s): Bendamustine, Multiple myeloma
Type: Publication Only
Background
Relapsed and refractory (R/R) multiple myeloma (MM) constitutes a specific and unmet medical need. Median survival ranges from as little as 6 to 9 months, and responses to treatment are characteristically short. In patients with R/R MM after therapy of bortezomib and/or immunomodulators (IMiDs) a bendamustine-based treatment can be used as “salvage”.
Aims
Examine efficiency of bendamustine-based chemotherapy in R/R MM patients.
Methods
In this retrospective analysis we have identified 32 patients with R/R MM by means of case research, who have been bendamustine-based treated at Hematological Clinics of Russian Federation since 2011. Median age was 67 years, the female/male ratio was 2.5:1. After in median 2 lines of prior therapy (range: 1-7) patients received in median 3 (range: 1-9) cycles of bendamustine-based therapy. Bendamustine dosage was 70-120 mg/m2/day on days 1 and 2 of a each 28-day cycle until progressive disease or intolerability. Bendamustine was administered as monotherapy in 12% of patients, whereas 88% received concomitant steroids (group Bendamustine (B), n=17). Bendamustine with dexamethasone and bortezomib or IMiDs (BBD, BRD, BTD) was evaluated in 15 patients with R/R MM (group Bendamustine-plus (B+)). Primary end point was overall response rate (ORR). Secondary end points were time to progression (TTP), overall survival (OS), and toxicity.
Results
ORR in both group was 59.2%: 22.2% partial response (6.7% in group B vs. 41.7% in group B+, ?<0.05), stable disease 37.0% (26.7% vs. 50.0%, accordingly, ?>0.05). Median TTP was 9.7 months (4.1 mo in group B vs. 7.2 mo in group B+, ?=0.02)) (pic). Median OS was 25.4 months in group B, but in group B+ median OS is not reached. Hematologic toxicity was in 25.0% of patients: grade 3/4 anemia was noted in 9.4%, grade 3/4 thrombocytopenia was noted in 6.3%, and grade 3/4 infections were noted in 21.9%.
Summary
Bendamustine-based therapy is active and well tolerated in patients with relapsed/refractory multiple myeloma.
Keyword(s): Bendamustine, Multiple myeloma

Abstract: PB1895
Type: Publication Only
Background
Relapsed and refractory (R/R) multiple myeloma (MM) constitutes a specific and unmet medical need. Median survival ranges from as little as 6 to 9 months, and responses to treatment are characteristically short. In patients with R/R MM after therapy of bortezomib and/or immunomodulators (IMiDs) a bendamustine-based treatment can be used as “salvage”.
Aims
Examine efficiency of bendamustine-based chemotherapy in R/R MM patients.
Methods
In this retrospective analysis we have identified 32 patients with R/R MM by means of case research, who have been bendamustine-based treated at Hematological Clinics of Russian Federation since 2011. Median age was 67 years, the female/male ratio was 2.5:1. After in median 2 lines of prior therapy (range: 1-7) patients received in median 3 (range: 1-9) cycles of bendamustine-based therapy. Bendamustine dosage was 70-120 mg/m2/day on days 1 and 2 of a each 28-day cycle until progressive disease or intolerability. Bendamustine was administered as monotherapy in 12% of patients, whereas 88% received concomitant steroids (group Bendamustine (B), n=17). Bendamustine with dexamethasone and bortezomib or IMiDs (BBD, BRD, BTD) was evaluated in 15 patients with R/R MM (group Bendamustine-plus (B+)). Primary end point was overall response rate (ORR). Secondary end points were time to progression (TTP), overall survival (OS), and toxicity.
Results
ORR in both group was 59.2%: 22.2% partial response (6.7% in group B vs. 41.7% in group B+, ?<0.05), stable disease 37.0% (26.7% vs. 50.0%, accordingly, ?>0.05). Median TTP was 9.7 months (4.1 mo in group B vs. 7.2 mo in group B+, ?=0.02)) (pic). Median OS was 25.4 months in group B, but in group B+ median OS is not reached. Hematologic toxicity was in 25.0% of patients: grade 3/4 anemia was noted in 9.4%, grade 3/4 thrombocytopenia was noted in 6.3%, and grade 3/4 infections were noted in 21.9%.
Summary
Bendamustine-based therapy is active and well tolerated in patients with relapsed/refractory multiple myeloma.
Keyword(s): Bendamustine, Multiple myeloma

Type: Publication Only
Background
Relapsed and refractory (R/R) multiple myeloma (MM) constitutes a specific and unmet medical need. Median survival ranges from as little as 6 to 9 months, and responses to treatment are characteristically short. In patients with R/R MM after therapy of bortezomib and/or immunomodulators (IMiDs) a bendamustine-based treatment can be used as “salvage”.
Aims
Examine efficiency of bendamustine-based chemotherapy in R/R MM patients.
Methods
In this retrospective analysis we have identified 32 patients with R/R MM by means of case research, who have been bendamustine-based treated at Hematological Clinics of Russian Federation since 2011. Median age was 67 years, the female/male ratio was 2.5:1. After in median 2 lines of prior therapy (range: 1-7) patients received in median 3 (range: 1-9) cycles of bendamustine-based therapy. Bendamustine dosage was 70-120 mg/m2/day on days 1 and 2 of a each 28-day cycle until progressive disease or intolerability. Bendamustine was administered as monotherapy in 12% of patients, whereas 88% received concomitant steroids (group Bendamustine (B), n=17). Bendamustine with dexamethasone and bortezomib or IMiDs (BBD, BRD, BTD) was evaluated in 15 patients with R/R MM (group Bendamustine-plus (B+)). Primary end point was overall response rate (ORR). Secondary end points were time to progression (TTP), overall survival (OS), and toxicity.
Results
ORR in both group was 59.2%: 22.2% partial response (6.7% in group B vs. 41.7% in group B+, ?<0.05), stable disease 37.0% (26.7% vs. 50.0%, accordingly, ?>0.05). Median TTP was 9.7 months (4.1 mo in group B vs. 7.2 mo in group B+, ?=0.02)) (pic). Median OS was 25.4 months in group B, but in group B+ median OS is not reached. Hematologic toxicity was in 25.0% of patients: grade 3/4 anemia was noted in 9.4%, grade 3/4 thrombocytopenia was noted in 6.3%, and grade 3/4 infections were noted in 21.9%.
Summary
Bendamustine-based therapy is active and well tolerated in patients with relapsed/refractory multiple myeloma.
Keyword(s): Bendamustine, Multiple myeloma

{{ help_message }}
{{filter}}


