TIME TO ONSET AND DURATION OF INDUCTION THERAPY AND ASSOCIATED FACTORS AMONG NEWLY DIAGNOSED MULTIPLE MYELOMA PATIENTS
(Abstract release date: 05/21/15)
EHA Library. Yusuf A. 06/12/15; 102797; PB1874
Disclosure(s): CDRG
Akeem Yusuf
Contributions
Contributions
Abstract
Abstract: PB1874
Type: Publication Only
Background
While early start of induction chemotherapy may improve treatment outcomes for patients with cancer, little is known about time to treatment onset and duration of induction therapy in newly diagnosed multiple myeloma (NDMM) patients.
Aims
We examined onset and duration of induction therapy and associated factors including age, gender, race, index year, stem cell therapy, and drug regimen in NDMM patients in the United States.
Methods
Using Medicare 20% data, we created a cohort of adult (≥18 years) NDMM patients (2008-2010) who initiated therapy with defined medications within 1 year of cancer diagnosis. Induction therapy regimens were categorized as bortezomib-based, immunomodulator (IMiD)-based, bortezomib-IMiD-based, or corticosteroid-based. Median number of days from diagnosis to therapy onset and of therapy duration were examined overall and for associated factors in a univariate approach using the Kruskal-Wallis test.
Results
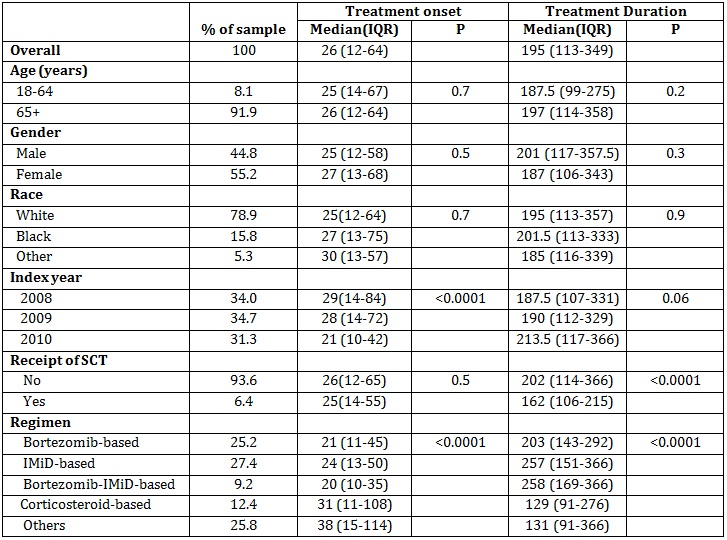
We identified 1455 NDMM patients. Overall, median (IQR) number of days to therapy onset from diagnosis and of therapy duration were 26 (12-64) and 195 (113-349), respectively. Neither days to onset nor duration varied significantly by age, sex, or race. Days to onset decreased from 29 to 21 days from 2008 to 2010, respectively (p<0.0001), but therapy duration was similar across years ranging from 188 to 214 days (p=0.06). Although days to onset were similar for patients who did (25 days) and did not (26 days) receive stem cell therapy (SCT; p=0.52), median therapy duration was 25% longer at 202 days for those who did not (p<0.0001). Onset was more rapid (21, 24, and 20 vs. 31 days) and duration longer (203, 257, and 258 vs. 129 days) for patients treated with bortezomib-, IMiD-, and bortezomib-IMiD-based regimens compared to those treated with corticosteroid-based regimens, respectively. Duration of therapy was longer for patients who did not receive SCT compared to those who did for bortezomib-based (209 vs. 156 days), IMiD-based (284 vs. 124 days), and bortezomib-IMiD-based (291 vs. 142 days) regimens, respectively.
Summary
We observed differences in time to onset and duration of induction therapy by index year, SCT status, and choice of regimen, and found no racial or sex differences. The clinical implications of these findings are unknown. Further studies are warranted to better understand the observed differences.
Keyword(s): Induction chemotherapy, Myeloma
Session topic: Publication Only
Type: Publication Only
Background
While early start of induction chemotherapy may improve treatment outcomes for patients with cancer, little is known about time to treatment onset and duration of induction therapy in newly diagnosed multiple myeloma (NDMM) patients.
Aims
We examined onset and duration of induction therapy and associated factors including age, gender, race, index year, stem cell therapy, and drug regimen in NDMM patients in the United States.
Methods
Using Medicare 20% data, we created a cohort of adult (≥18 years) NDMM patients (2008-2010) who initiated therapy with defined medications within 1 year of cancer diagnosis. Induction therapy regimens were categorized as bortezomib-based, immunomodulator (IMiD)-based, bortezomib-IMiD-based, or corticosteroid-based. Median number of days from diagnosis to therapy onset and of therapy duration were examined overall and for associated factors in a univariate approach using the Kruskal-Wallis test.
Results
We identified 1455 NDMM patients. Overall, median (IQR) number of days to therapy onset from diagnosis and of therapy duration were 26 (12-64) and 195 (113-349), respectively. Neither days to onset nor duration varied significantly by age, sex, or race. Days to onset decreased from 29 to 21 days from 2008 to 2010, respectively (p<0.0001), but therapy duration was similar across years ranging from 188 to 214 days (p=0.06). Although days to onset were similar for patients who did (25 days) and did not (26 days) receive stem cell therapy (SCT; p=0.52), median therapy duration was 25% longer at 202 days for those who did not (p<0.0001). Onset was more rapid (21, 24, and 20 vs. 31 days) and duration longer (203, 257, and 258 vs. 129 days) for patients treated with bortezomib-, IMiD-, and bortezomib-IMiD-based regimens compared to those treated with corticosteroid-based regimens, respectively. Duration of therapy was longer for patients who did not receive SCT compared to those who did for bortezomib-based (209 vs. 156 days), IMiD-based (284 vs. 124 days), and bortezomib-IMiD-based (291 vs. 142 days) regimens, respectively.
Summary
We observed differences in time to onset and duration of induction therapy by index year, SCT status, and choice of regimen, and found no racial or sex differences. The clinical implications of these findings are unknown. Further studies are warranted to better understand the observed differences.
Keyword(s): Induction chemotherapy, Myeloma

Session topic: Publication Only
Abstract: PB1874
Type: Publication Only
Background
While early start of induction chemotherapy may improve treatment outcomes for patients with cancer, little is known about time to treatment onset and duration of induction therapy in newly diagnosed multiple myeloma (NDMM) patients.
Aims
We examined onset and duration of induction therapy and associated factors including age, gender, race, index year, stem cell therapy, and drug regimen in NDMM patients in the United States.
Methods
Using Medicare 20% data, we created a cohort of adult (≥18 years) NDMM patients (2008-2010) who initiated therapy with defined medications within 1 year of cancer diagnosis. Induction therapy regimens were categorized as bortezomib-based, immunomodulator (IMiD)-based, bortezomib-IMiD-based, or corticosteroid-based. Median number of days from diagnosis to therapy onset and of therapy duration were examined overall and for associated factors in a univariate approach using the Kruskal-Wallis test.
Results
We identified 1455 NDMM patients. Overall, median (IQR) number of days to therapy onset from diagnosis and of therapy duration were 26 (12-64) and 195 (113-349), respectively. Neither days to onset nor duration varied significantly by age, sex, or race. Days to onset decreased from 29 to 21 days from 2008 to 2010, respectively (p<0.0001), but therapy duration was similar across years ranging from 188 to 214 days (p=0.06). Although days to onset were similar for patients who did (25 days) and did not (26 days) receive stem cell therapy (SCT; p=0.52), median therapy duration was 25% longer at 202 days for those who did not (p<0.0001). Onset was more rapid (21, 24, and 20 vs. 31 days) and duration longer (203, 257, and 258 vs. 129 days) for patients treated with bortezomib-, IMiD-, and bortezomib-IMiD-based regimens compared to those treated with corticosteroid-based regimens, respectively. Duration of therapy was longer for patients who did not receive SCT compared to those who did for bortezomib-based (209 vs. 156 days), IMiD-based (284 vs. 124 days), and bortezomib-IMiD-based (291 vs. 142 days) regimens, respectively.
Summary
We observed differences in time to onset and duration of induction therapy by index year, SCT status, and choice of regimen, and found no racial or sex differences. The clinical implications of these findings are unknown. Further studies are warranted to better understand the observed differences.
Keyword(s): Induction chemotherapy, Myeloma

Session topic: Publication Only
Type: Publication Only
Background
While early start of induction chemotherapy may improve treatment outcomes for patients with cancer, little is known about time to treatment onset and duration of induction therapy in newly diagnosed multiple myeloma (NDMM) patients.
Aims
We examined onset and duration of induction therapy and associated factors including age, gender, race, index year, stem cell therapy, and drug regimen in NDMM patients in the United States.
Methods
Using Medicare 20% data, we created a cohort of adult (≥18 years) NDMM patients (2008-2010) who initiated therapy with defined medications within 1 year of cancer diagnosis. Induction therapy regimens were categorized as bortezomib-based, immunomodulator (IMiD)-based, bortezomib-IMiD-based, or corticosteroid-based. Median number of days from diagnosis to therapy onset and of therapy duration were examined overall and for associated factors in a univariate approach using the Kruskal-Wallis test.
Results
We identified 1455 NDMM patients. Overall, median (IQR) number of days to therapy onset from diagnosis and of therapy duration were 26 (12-64) and 195 (113-349), respectively. Neither days to onset nor duration varied significantly by age, sex, or race. Days to onset decreased from 29 to 21 days from 2008 to 2010, respectively (p<0.0001), but therapy duration was similar across years ranging from 188 to 214 days (p=0.06). Although days to onset were similar for patients who did (25 days) and did not (26 days) receive stem cell therapy (SCT; p=0.52), median therapy duration was 25% longer at 202 days for those who did not (p<0.0001). Onset was more rapid (21, 24, and 20 vs. 31 days) and duration longer (203, 257, and 258 vs. 129 days) for patients treated with bortezomib-, IMiD-, and bortezomib-IMiD-based regimens compared to those treated with corticosteroid-based regimens, respectively. Duration of therapy was longer for patients who did not receive SCT compared to those who did for bortezomib-based (209 vs. 156 days), IMiD-based (284 vs. 124 days), and bortezomib-IMiD-based (291 vs. 142 days) regimens, respectively.
Summary
We observed differences in time to onset and duration of induction therapy by index year, SCT status, and choice of regimen, and found no racial or sex differences. The clinical implications of these findings are unknown. Further studies are warranted to better understand the observed differences.
Keyword(s): Induction chemotherapy, Myeloma

Session topic: Publication Only
{{ help_message }}
{{filter}}


