
Contributions
Type: Publication Only
Background
Immune dysregulation with autoimmune phenomena, especially autoimmune haemolytic anaemia (AIHA), is a common complication over the lifetime of CLL patients. Therapy-related haemolysis in CLL was first described in 1966 in patients treated with radiotherapy or alkylating agents. Drug-induced haemolytic anaemia mostly related to the use of fludarabine has been often reported, even if the use of monoclonal antibody with chemotherapeutic agents could reduce the incidence of haemolytic episodes. Bendamustine is an alkylating agent composed by benzimidazole ring which is similar to some purine analogs; for these reasons the haemolysis generated by bendamustine should be similar to that induced by fludarabine. To date, this complication was rarely reported in association with bendamustine.
Aims
We reported the experience of 4 Italian haematological centres focusing on AIHA during treatment with bendamustine plus rituximab (BR) in patients affected by CLL.
Methods
We included in the study all CLL patients who underwent BR treatment as front-line or successive treatment for progressive disease. All patients who experienced AIHA during or after BR treatment were described. AIHA was diagnosed as reduction of haemoglobin level with positivity of haemolysis tests.
Results
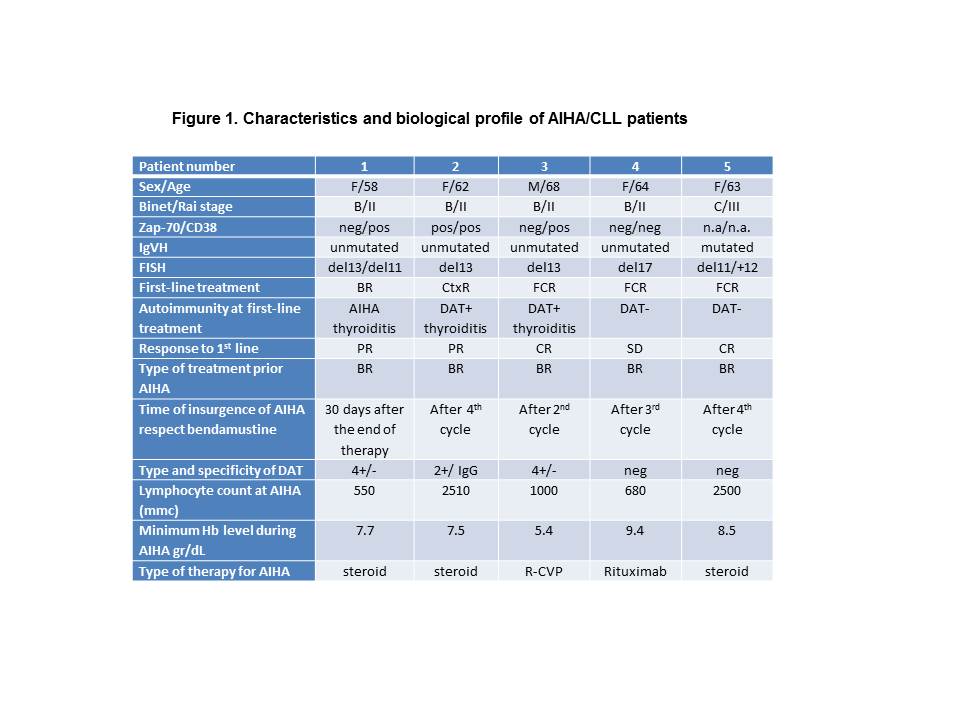
One hundred-thirty CLL patients were treated with BR in our four centres. Of these, 61 patients were treated for progressive CLL as first-line therapy and 69 patients as second-line therapy. No patient experienced autoimmune phenomena when bendamustine was used with rituximab as part of front-line therapy. On the opposite in CLL patients treated with BR as second-line therapy, 5 episodes of AIHA were reported as shown in Figure 1. The prevalence of bendamustine related AIHA in patients who underwent to BR treatment as second line therapy was 7.2%. Three of five patients who experienced AIHA had positive DAT, AIHA or immunological autoantibodies at the onset of CLL. Three cases used fludarabine based regimens as front-line therapy. Three of five patients developed DAT positive AIHA; low median lymphocytes count (1000/mmc) and the use of rituximab could justified DAT negative AIHA in two patients. The onset of AIHA was observed during the BR treatment in 4 patients and at the end of planned therapy in 1 patient. The course of AIHA was mild, it responded to steroid or rituximab therapy and it was solved in a few days.
Summary
All 5 patients experienced bendamustine related AIHA during or after BR treatment as second line chemotherapy. Our experience confirms the literature data in which 9 of 10 cases of bendamustine related AIHA in CLL patients reported, appeared after second or further treatment. Patients who experienced previous autoimmune phenomena or underwent fludarabine as front-line therapy, probably for its similitude in the drug structure to bendamustine, showed an increase risk of AIHA. AIHA is probably due to depletion of CD4 cells which can lead to failure to control auto-reactive T-cells that are free to create autoimmunity. The course of bendamustine related AIHA was mild, it could be quickly solved in a few days with the use of immunosuppressive agents as steroid or monoclonal antibodies. In conclusion BR is safe and effective regimen in CLL patients, a care observation should be taken in patients in which BR was used as second or further line therapy. Previous autoimmune phenomena, prior use of fludarabine and CD4 depletion could explain the increased incidence of bendamustine related AIHA.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Bendamustine, Chronic lymphocytic leukemia, Rituximab
Session topic: Publication Only
Type: Publication Only
Background
Immune dysregulation with autoimmune phenomena, especially autoimmune haemolytic anaemia (AIHA), is a common complication over the lifetime of CLL patients. Therapy-related haemolysis in CLL was first described in 1966 in patients treated with radiotherapy or alkylating agents. Drug-induced haemolytic anaemia mostly related to the use of fludarabine has been often reported, even if the use of monoclonal antibody with chemotherapeutic agents could reduce the incidence of haemolytic episodes. Bendamustine is an alkylating agent composed by benzimidazole ring which is similar to some purine analogs; for these reasons the haemolysis generated by bendamustine should be similar to that induced by fludarabine. To date, this complication was rarely reported in association with bendamustine.
Aims
We reported the experience of 4 Italian haematological centres focusing on AIHA during treatment with bendamustine plus rituximab (BR) in patients affected by CLL.
Methods
We included in the study all CLL patients who underwent BR treatment as front-line or successive treatment for progressive disease. All patients who experienced AIHA during or after BR treatment were described. AIHA was diagnosed as reduction of haemoglobin level with positivity of haemolysis tests.
Results
One hundred-thirty CLL patients were treated with BR in our four centres. Of these, 61 patients were treated for progressive CLL as first-line therapy and 69 patients as second-line therapy. No patient experienced autoimmune phenomena when bendamustine was used with rituximab as part of front-line therapy. On the opposite in CLL patients treated with BR as second-line therapy, 5 episodes of AIHA were reported as shown in Figure 1. The prevalence of bendamustine related AIHA in patients who underwent to BR treatment as second line therapy was 7.2%. Three of five patients who experienced AIHA had positive DAT, AIHA or immunological autoantibodies at the onset of CLL. Three cases used fludarabine based regimens as front-line therapy. Three of five patients developed DAT positive AIHA; low median lymphocytes count (1000/mmc) and the use of rituximab could justified DAT negative AIHA in two patients. The onset of AIHA was observed during the BR treatment in 4 patients and at the end of planned therapy in 1 patient. The course of AIHA was mild, it responded to steroid or rituximab therapy and it was solved in a few days.
Summary
All 5 patients experienced bendamustine related AIHA during or after BR treatment as second line chemotherapy. Our experience confirms the literature data in which 9 of 10 cases of bendamustine related AIHA in CLL patients reported, appeared after second or further treatment. Patients who experienced previous autoimmune phenomena or underwent fludarabine as front-line therapy, probably for its similitude in the drug structure to bendamustine, showed an increase risk of AIHA. AIHA is probably due to depletion of CD4 cells which can lead to failure to control auto-reactive T-cells that are free to create autoimmunity. The course of bendamustine related AIHA was mild, it could be quickly solved in a few days with the use of immunosuppressive agents as steroid or monoclonal antibodies. In conclusion BR is safe and effective regimen in CLL patients, a care observation should be taken in patients in which BR was used as second or further line therapy. Previous autoimmune phenomena, prior use of fludarabine and CD4 depletion could explain the increased incidence of bendamustine related AIHA.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Bendamustine, Chronic lymphocytic leukemia, Rituximab

Session topic: Publication Only


