GENERIC IMATINIB MESYLATE IS AS EFFECTIVE AS ORIGINAL GLIVEC IN THE MANAGEMENT OF CML
(Abstract release date: 05/21/15)
EHA Library. C.Haznedaroglu I. 06/12/15; 102756; PB1742

Ibrahim C.Haznedaroglu
Contributions
Contributions
Abstract
Abstract: PB1742
Type: Publication Only
Background
Unsustainable drug prices in chronic myeloid leukemia (CML) and cancer may be causing harm to patients. Advocating for lower drug prices is a necessity to save the lives of patients who cannot afford them (Experts in CML. Blood. 2013; 121(22):4439-4442). The patent date of imatinib mesylate in USA has just expired in January 2015. Patent expiration dates for imatinib may be different in different countries/regions. In Turkey, generic imatinib preparations are currently present. The only concern for generic imatinib is its efficacy over the original drug, Glivec or Gleevec.
Aims
The aim of this multi-center study is to assess the efficacy of generic imatinib over Glivec in terms of hematological, cytogenetic, and molecular responses in CML.
Methods
This work is designed as multicenter and retrospective study.
Results
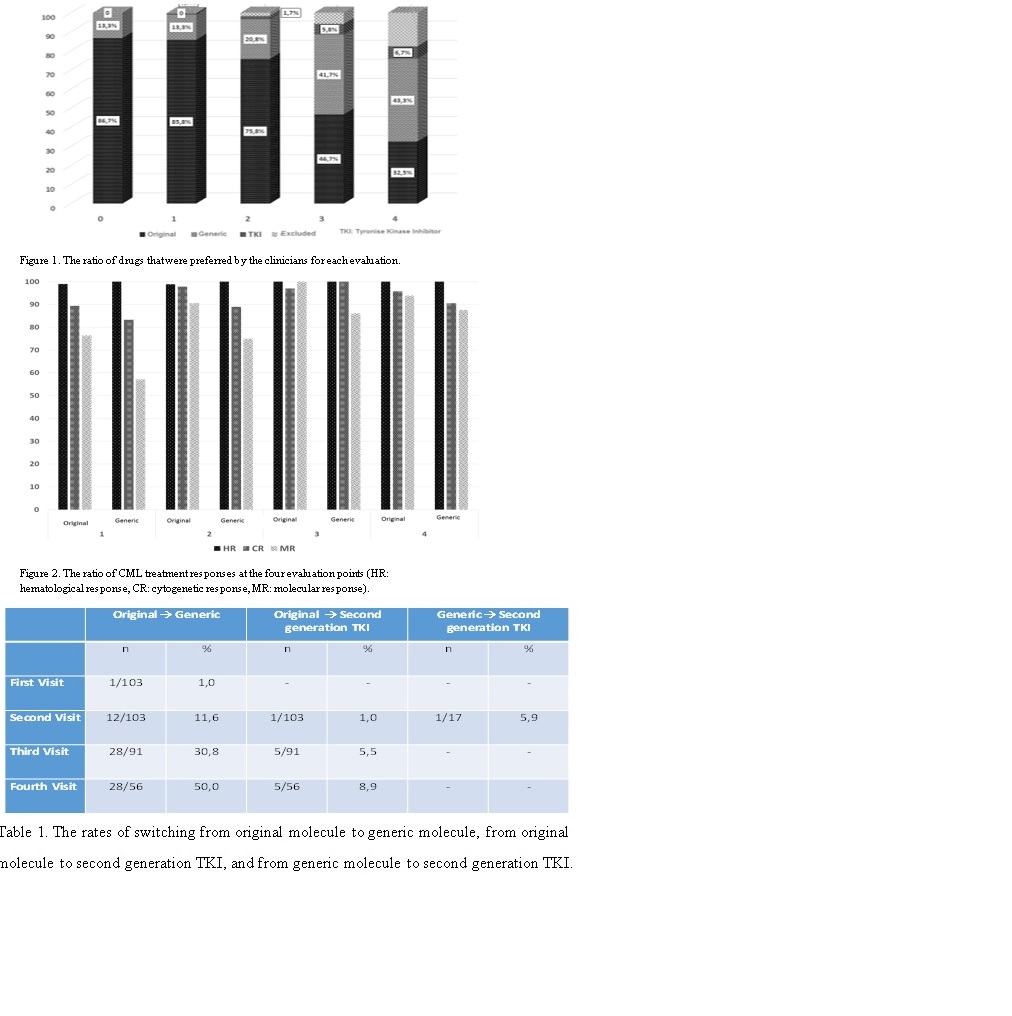
In this study, the retrospective data of 120 Turkish CML patients receiving imatinib from six different CML centers across Turkey were analyzed (68 females, 56.7%; 52 males, 43.3%). The mean age was 53 years (21-81). The most frequent ECOG performance status was “0” (65%). The distribution of genders between centers was similar. At the study onset, 86.7% of the patients (n=104) were using original molecules, and 13.3% of the patients (n=16) were using generic molecules. Original (86.7%), 16 generic imatinib mesylate. The patients were evaluated at 4 different time points for change of medication and efficacy. The mean period between each evaluation was 9 months. Initial evaluation showed that a patient who was using only original molecule, switched to second generation tyrosine kinase inhibitor (TKI) treatment. In this period, hematological response (HR) was observed in 99.2% of the patients, cytogenetic response (CR) was observed in 88.7% of the patients (47 of 53), and molecular response (MR) was observed in 75% of the patients. For each evaluation, the ratio of drugs that were preferred by the clinicians is shown in Figure 1. According to Figure 1, clinicians had a tendency to prefer generic molecules in each sequent visit, and this change rate was significant (p<0.001). The rate of using generic molecules was 13.3% in the initial evaluation, 20.8% in the second visit, 41.7% in the third visit, and 43.3% in the fourth visit. The rates of switching from original molecule to generic molecule, from original molecule to second generation TKI, and from generic molecule to second generation TKI are shown in Table 1. Accordingly, 11 patients, who were using original molecules during all cohort, switched to second generation TKI. On the other hand, only 1 patient, who was using generic molecules, switched to second generation TKI. Response to treatment is shown in Figure 2.
Summary
Therefore, we did not find any significant difference in HR, CR, and MR for original and generic drugs in each visit. Based on this data, generic imatinib mesylate is as effective as original Glivec in the management of CML.
Keyword(s): Imatinib
Session topic: Publication Only
Type: Publication Only
Background
Unsustainable drug prices in chronic myeloid leukemia (CML) and cancer may be causing harm to patients. Advocating for lower drug prices is a necessity to save the lives of patients who cannot afford them (Experts in CML. Blood. 2013; 121(22):4439-4442). The patent date of imatinib mesylate in USA has just expired in January 2015. Patent expiration dates for imatinib may be different in different countries/regions. In Turkey, generic imatinib preparations are currently present. The only concern for generic imatinib is its efficacy over the original drug, Glivec or Gleevec.
Aims
The aim of this multi-center study is to assess the efficacy of generic imatinib over Glivec in terms of hematological, cytogenetic, and molecular responses in CML.
Methods
This work is designed as multicenter and retrospective study.
Results
In this study, the retrospective data of 120 Turkish CML patients receiving imatinib from six different CML centers across Turkey were analyzed (68 females, 56.7%; 52 males, 43.3%). The mean age was 53 years (21-81). The most frequent ECOG performance status was “0” (65%). The distribution of genders between centers was similar. At the study onset, 86.7% of the patients (n=104) were using original molecules, and 13.3% of the patients (n=16) were using generic molecules. Original (86.7%), 16 generic imatinib mesylate. The patients were evaluated at 4 different time points for change of medication and efficacy. The mean period between each evaluation was 9 months. Initial evaluation showed that a patient who was using only original molecule, switched to second generation tyrosine kinase inhibitor (TKI) treatment. In this period, hematological response (HR) was observed in 99.2% of the patients, cytogenetic response (CR) was observed in 88.7% of the patients (47 of 53), and molecular response (MR) was observed in 75% of the patients. For each evaluation, the ratio of drugs that were preferred by the clinicians is shown in Figure 1. According to Figure 1, clinicians had a tendency to prefer generic molecules in each sequent visit, and this change rate was significant (p<0.001). The rate of using generic molecules was 13.3% in the initial evaluation, 20.8% in the second visit, 41.7% in the third visit, and 43.3% in the fourth visit. The rates of switching from original molecule to generic molecule, from original molecule to second generation TKI, and from generic molecule to second generation TKI are shown in Table 1. Accordingly, 11 patients, who were using original molecules during all cohort, switched to second generation TKI. On the other hand, only 1 patient, who was using generic molecules, switched to second generation TKI. Response to treatment is shown in Figure 2.
Summary
Therefore, we did not find any significant difference in HR, CR, and MR for original and generic drugs in each visit. Based on this data, generic imatinib mesylate is as effective as original Glivec in the management of CML.
Keyword(s): Imatinib

Session topic: Publication Only
Abstract: PB1742
Type: Publication Only
Background
Unsustainable drug prices in chronic myeloid leukemia (CML) and cancer may be causing harm to patients. Advocating for lower drug prices is a necessity to save the lives of patients who cannot afford them (Experts in CML. Blood. 2013; 121(22):4439-4442). The patent date of imatinib mesylate in USA has just expired in January 2015. Patent expiration dates for imatinib may be different in different countries/regions. In Turkey, generic imatinib preparations are currently present. The only concern for generic imatinib is its efficacy over the original drug, Glivec or Gleevec.
Aims
The aim of this multi-center study is to assess the efficacy of generic imatinib over Glivec in terms of hematological, cytogenetic, and molecular responses in CML.
Methods
This work is designed as multicenter and retrospective study.
Results
In this study, the retrospective data of 120 Turkish CML patients receiving imatinib from six different CML centers across Turkey were analyzed (68 females, 56.7%; 52 males, 43.3%). The mean age was 53 years (21-81). The most frequent ECOG performance status was “0” (65%). The distribution of genders between centers was similar. At the study onset, 86.7% of the patients (n=104) were using original molecules, and 13.3% of the patients (n=16) were using generic molecules. Original (86.7%), 16 generic imatinib mesylate. The patients were evaluated at 4 different time points for change of medication and efficacy. The mean period between each evaluation was 9 months. Initial evaluation showed that a patient who was using only original molecule, switched to second generation tyrosine kinase inhibitor (TKI) treatment. In this period, hematological response (HR) was observed in 99.2% of the patients, cytogenetic response (CR) was observed in 88.7% of the patients (47 of 53), and molecular response (MR) was observed in 75% of the patients. For each evaluation, the ratio of drugs that were preferred by the clinicians is shown in Figure 1. According to Figure 1, clinicians had a tendency to prefer generic molecules in each sequent visit, and this change rate was significant (p<0.001). The rate of using generic molecules was 13.3% in the initial evaluation, 20.8% in the second visit, 41.7% in the third visit, and 43.3% in the fourth visit. The rates of switching from original molecule to generic molecule, from original molecule to second generation TKI, and from generic molecule to second generation TKI are shown in Table 1. Accordingly, 11 patients, who were using original molecules during all cohort, switched to second generation TKI. On the other hand, only 1 patient, who was using generic molecules, switched to second generation TKI. Response to treatment is shown in Figure 2.
Summary
Therefore, we did not find any significant difference in HR, CR, and MR for original and generic drugs in each visit. Based on this data, generic imatinib mesylate is as effective as original Glivec in the management of CML.
Keyword(s): Imatinib

Session topic: Publication Only
Type: Publication Only
Background
Unsustainable drug prices in chronic myeloid leukemia (CML) and cancer may be causing harm to patients. Advocating for lower drug prices is a necessity to save the lives of patients who cannot afford them (Experts in CML. Blood. 2013; 121(22):4439-4442). The patent date of imatinib mesylate in USA has just expired in January 2015. Patent expiration dates for imatinib may be different in different countries/regions. In Turkey, generic imatinib preparations are currently present. The only concern for generic imatinib is its efficacy over the original drug, Glivec or Gleevec.
Aims
The aim of this multi-center study is to assess the efficacy of generic imatinib over Glivec in terms of hematological, cytogenetic, and molecular responses in CML.
Methods
This work is designed as multicenter and retrospective study.
Results
In this study, the retrospective data of 120 Turkish CML patients receiving imatinib from six different CML centers across Turkey were analyzed (68 females, 56.7%; 52 males, 43.3%). The mean age was 53 years (21-81). The most frequent ECOG performance status was “0” (65%). The distribution of genders between centers was similar. At the study onset, 86.7% of the patients (n=104) were using original molecules, and 13.3% of the patients (n=16) were using generic molecules. Original (86.7%), 16 generic imatinib mesylate. The patients were evaluated at 4 different time points for change of medication and efficacy. The mean period between each evaluation was 9 months. Initial evaluation showed that a patient who was using only original molecule, switched to second generation tyrosine kinase inhibitor (TKI) treatment. In this period, hematological response (HR) was observed in 99.2% of the patients, cytogenetic response (CR) was observed in 88.7% of the patients (47 of 53), and molecular response (MR) was observed in 75% of the patients. For each evaluation, the ratio of drugs that were preferred by the clinicians is shown in Figure 1. According to Figure 1, clinicians had a tendency to prefer generic molecules in each sequent visit, and this change rate was significant (p<0.001). The rate of using generic molecules was 13.3% in the initial evaluation, 20.8% in the second visit, 41.7% in the third visit, and 43.3% in the fourth visit. The rates of switching from original molecule to generic molecule, from original molecule to second generation TKI, and from generic molecule to second generation TKI are shown in Table 1. Accordingly, 11 patients, who were using original molecules during all cohort, switched to second generation TKI. On the other hand, only 1 patient, who was using generic molecules, switched to second generation TKI. Response to treatment is shown in Figure 2.
Summary
Therefore, we did not find any significant difference in HR, CR, and MR for original and generic drugs in each visit. Based on this data, generic imatinib mesylate is as effective as original Glivec in the management of CML.
Keyword(s): Imatinib

Session topic: Publication Only
{{ help_message }}
{{filter}}


