GEOGRAPHICAL DIFFERENCES IN IRON OVERLOAD AND IRON CHELATION PRACTICES IN ANEMIA PATIENTS: BASELINE RESULTS FROM THE TRANSFUSIONAL HEMOSIDEROSIS REGISTRY STUDY (TORS)
(Abstract release date: 05/21/15)
EHA Library. Siritanaratkul N. 06/12/15; 102751; PB2002
Disclosure(s): Mahidol UniversityFaculty of Medicine Siriraj Hospital

Noppadol Siritanaratkul
Contributions
Contributions
Abstract
Abstract: PB2002
Type: Publication Only
Background
Data describing transfusion practices and diagnoses of iron overload as well as iron chelation in patients with anemia across geographical regions are limited. A prospective epidemiological study may provide further insights into clinical practices for these conditions.
Aims
This prospective, multinational, non-interventional study, aimed to (1) evaluate the differences in baseline characteristics among early diagnosed patients with anemia requiring chronic transfusion therapy; (2) assess the extent of iron overload and patterns of care surrounding the use of iron chelation therapy in different geographical regions.
Methods
Patients aged >2 years requiring chronic transfusion therapy with newly diagnosed anemias (<12 months from diagnosis), including thalassemia, low and intermediate-1 myelodysplastic syndromes (MDS), aplastic anemia (AA), Diamond-Blackfan anemia (DBA) and other transfusion-dependent anemias were enrolled. Patients were recruited from Turkey, Russia and South Africa as well as from countries within the Asia-Pacific and Middle East regions. Patients with secondary or therapy-related MDS; intermediate-2 or high-risk MDS; or acute leukemia were excluded. Patients were evaluated at baseline and at follow-up visits according to the standard practice for up to 3 years or until death. At baseline, patient demographics and details of iron overload and iron chelation therapy use were assessed. Transfusional hemosiderosis was defined as ≥1 serum ferritin measurement >1000 ng/mL after onset of transfusion therapy; or a liver iron concentration (LIC) >2 mg Fe/g dry weight; or if serum ferritin or LIC measurements were not available, evidence of ≥20 units of red blood cell transfusions.
Results
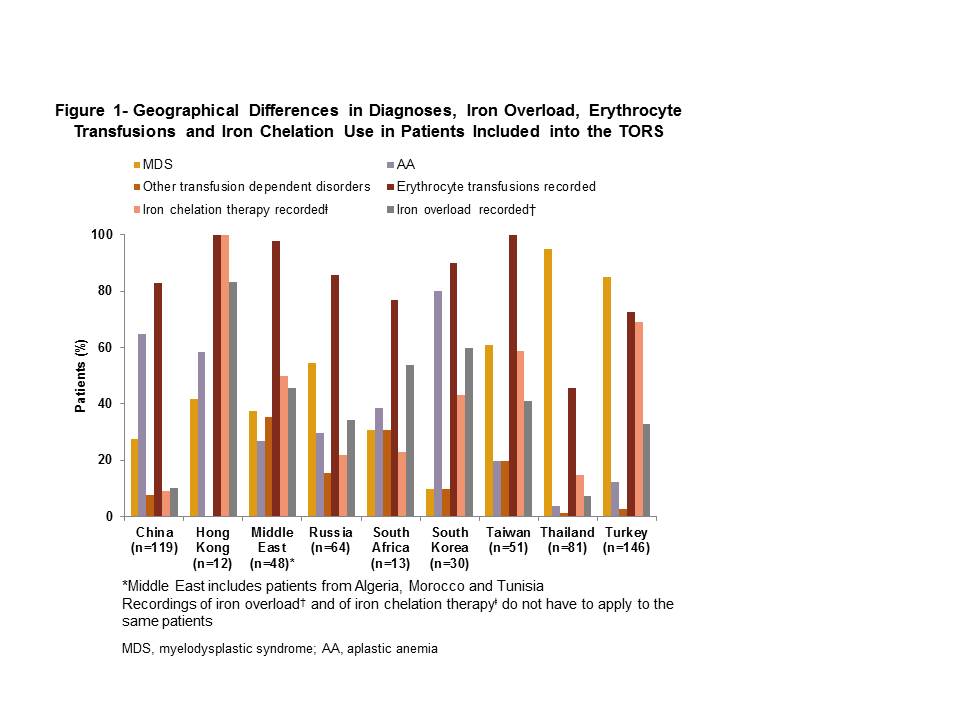
Of the 564 patients (including patients aged ≤18 years, n=57) recruited, majority had a diagnosis of MDS (58.5%, n=330), followed by AA (31.2%, n=176), other transfusion-dependent disorders (6.2%, n=35), β-thalassemia major (2.0%, n=11), β-thalassemia intermedia (1.1%, n=6), sickle cell anemia (0.5%, n=3) and DBA (0.4%, n=2). The mean age (±SD) of the patients was 51.9±23.87 years (range, 2-92); 49.5% (n=279) were male. At study entry, >70% of patients in all countries, except Thailand (45.7%), were receiving transfusion therapy. Overall, 29.4% (166/564) patients and 36.8% (21/57) pediatric patients were assessed as having iron overload during the study; extent of iron overload across countries varied and ranged from 7.4% (6/81) in Thailand to 83.3% (10/12) in Hong Kong. Patients receiving drug therapy only were less likely to be assessed for iron overload (1.2% [1/86]) than patients receiving erythrocyte transfusion (33.0% [33/100]) or erythrocyte transfusion and drug therapy (38.1% [131/344]). Overall, 39% of the population received iron chelation therapy and ranged from 9.2% (11/119) in China to 100.0% (12/12) in Hong Kong. Despite receiving iron chelation therapy, 56.4% (124/220 patients) were documented as having iron overload (Figure 1).
Summary
In this large observational study, MDS was the most common disease type enrolled. Majority of patients received transfusion therapy across countries; consequently there was a proportion of patients that had iron overload. Despite a large percent of patients receiving transfusion therapy and having developed iron overload, there was a broad spectrum of iron chelation use. Notably, over half of patients still had iron overload despite receiving chelation therapy. Overall, these results suggest that iron overload management practices may still be suboptimal in many parts of the world. Further studies are required to clearly understand these geographical differences in management of transfusion–dependent anemias.
Keyword(s): Clinical data, Iron chelation, Iron overload, Transfusion
Session topic: Publication Only
Type: Publication Only
Background
Data describing transfusion practices and diagnoses of iron overload as well as iron chelation in patients with anemia across geographical regions are limited. A prospective epidemiological study may provide further insights into clinical practices for these conditions.
Aims
This prospective, multinational, non-interventional study, aimed to (1) evaluate the differences in baseline characteristics among early diagnosed patients with anemia requiring chronic transfusion therapy; (2) assess the extent of iron overload and patterns of care surrounding the use of iron chelation therapy in different geographical regions.
Methods
Patients aged >2 years requiring chronic transfusion therapy with newly diagnosed anemias (<12 months from diagnosis), including thalassemia, low and intermediate-1 myelodysplastic syndromes (MDS), aplastic anemia (AA), Diamond-Blackfan anemia (DBA) and other transfusion-dependent anemias were enrolled. Patients were recruited from Turkey, Russia and South Africa as well as from countries within the Asia-Pacific and Middle East regions. Patients with secondary or therapy-related MDS; intermediate-2 or high-risk MDS; or acute leukemia were excluded. Patients were evaluated at baseline and at follow-up visits according to the standard practice for up to 3 years or until death. At baseline, patient demographics and details of iron overload and iron chelation therapy use were assessed. Transfusional hemosiderosis was defined as ≥1 serum ferritin measurement >1000 ng/mL after onset of transfusion therapy; or a liver iron concentration (LIC) >2 mg Fe/g dry weight; or if serum ferritin or LIC measurements were not available, evidence of ≥20 units of red blood cell transfusions.
Results
Of the 564 patients (including patients aged ≤18 years, n=57) recruited, majority had a diagnosis of MDS (58.5%, n=330), followed by AA (31.2%, n=176), other transfusion-dependent disorders (6.2%, n=35), β-thalassemia major (2.0%, n=11), β-thalassemia intermedia (1.1%, n=6), sickle cell anemia (0.5%, n=3) and DBA (0.4%, n=2). The mean age (±SD) of the patients was 51.9±23.87 years (range, 2-92); 49.5% (n=279) were male. At study entry, >70% of patients in all countries, except Thailand (45.7%), were receiving transfusion therapy. Overall, 29.4% (166/564) patients and 36.8% (21/57) pediatric patients were assessed as having iron overload during the study; extent of iron overload across countries varied and ranged from 7.4% (6/81) in Thailand to 83.3% (10/12) in Hong Kong. Patients receiving drug therapy only were less likely to be assessed for iron overload (1.2% [1/86]) than patients receiving erythrocyte transfusion (33.0% [33/100]) or erythrocyte transfusion and drug therapy (38.1% [131/344]). Overall, 39% of the population received iron chelation therapy and ranged from 9.2% (11/119) in China to 100.0% (12/12) in Hong Kong. Despite receiving iron chelation therapy, 56.4% (124/220 patients) were documented as having iron overload (Figure 1).
Summary
In this large observational study, MDS was the most common disease type enrolled. Majority of patients received transfusion therapy across countries; consequently there was a proportion of patients that had iron overload. Despite a large percent of patients receiving transfusion therapy and having developed iron overload, there was a broad spectrum of iron chelation use. Notably, over half of patients still had iron overload despite receiving chelation therapy. Overall, these results suggest that iron overload management practices may still be suboptimal in many parts of the world. Further studies are required to clearly understand these geographical differences in management of transfusion–dependent anemias.
Keyword(s): Clinical data, Iron chelation, Iron overload, Transfusion

Session topic: Publication Only
Abstract: PB2002
Type: Publication Only
Background
Data describing transfusion practices and diagnoses of iron overload as well as iron chelation in patients with anemia across geographical regions are limited. A prospective epidemiological study may provide further insights into clinical practices for these conditions.
Aims
This prospective, multinational, non-interventional study, aimed to (1) evaluate the differences in baseline characteristics among early diagnosed patients with anemia requiring chronic transfusion therapy; (2) assess the extent of iron overload and patterns of care surrounding the use of iron chelation therapy in different geographical regions.
Methods
Patients aged >2 years requiring chronic transfusion therapy with newly diagnosed anemias (<12 months from diagnosis), including thalassemia, low and intermediate-1 myelodysplastic syndromes (MDS), aplastic anemia (AA), Diamond-Blackfan anemia (DBA) and other transfusion-dependent anemias were enrolled. Patients were recruited from Turkey, Russia and South Africa as well as from countries within the Asia-Pacific and Middle East regions. Patients with secondary or therapy-related MDS; intermediate-2 or high-risk MDS; or acute leukemia were excluded. Patients were evaluated at baseline and at follow-up visits according to the standard practice for up to 3 years or until death. At baseline, patient demographics and details of iron overload and iron chelation therapy use were assessed. Transfusional hemosiderosis was defined as ≥1 serum ferritin measurement >1000 ng/mL after onset of transfusion therapy; or a liver iron concentration (LIC) >2 mg Fe/g dry weight; or if serum ferritin or LIC measurements were not available, evidence of ≥20 units of red blood cell transfusions.
Results
Of the 564 patients (including patients aged ≤18 years, n=57) recruited, majority had a diagnosis of MDS (58.5%, n=330), followed by AA (31.2%, n=176), other transfusion-dependent disorders (6.2%, n=35), β-thalassemia major (2.0%, n=11), β-thalassemia intermedia (1.1%, n=6), sickle cell anemia (0.5%, n=3) and DBA (0.4%, n=2). The mean age (±SD) of the patients was 51.9±23.87 years (range, 2-92); 49.5% (n=279) were male. At study entry, >70% of patients in all countries, except Thailand (45.7%), were receiving transfusion therapy. Overall, 29.4% (166/564) patients and 36.8% (21/57) pediatric patients were assessed as having iron overload during the study; extent of iron overload across countries varied and ranged from 7.4% (6/81) in Thailand to 83.3% (10/12) in Hong Kong. Patients receiving drug therapy only were less likely to be assessed for iron overload (1.2% [1/86]) than patients receiving erythrocyte transfusion (33.0% [33/100]) or erythrocyte transfusion and drug therapy (38.1% [131/344]). Overall, 39% of the population received iron chelation therapy and ranged from 9.2% (11/119) in China to 100.0% (12/12) in Hong Kong. Despite receiving iron chelation therapy, 56.4% (124/220 patients) were documented as having iron overload (Figure 1).
Summary
In this large observational study, MDS was the most common disease type enrolled. Majority of patients received transfusion therapy across countries; consequently there was a proportion of patients that had iron overload. Despite a large percent of patients receiving transfusion therapy and having developed iron overload, there was a broad spectrum of iron chelation use. Notably, over half of patients still had iron overload despite receiving chelation therapy. Overall, these results suggest that iron overload management practices may still be suboptimal in many parts of the world. Further studies are required to clearly understand these geographical differences in management of transfusion–dependent anemias.
Keyword(s): Clinical data, Iron chelation, Iron overload, Transfusion

Session topic: Publication Only
Type: Publication Only
Background
Data describing transfusion practices and diagnoses of iron overload as well as iron chelation in patients with anemia across geographical regions are limited. A prospective epidemiological study may provide further insights into clinical practices for these conditions.
Aims
This prospective, multinational, non-interventional study, aimed to (1) evaluate the differences in baseline characteristics among early diagnosed patients with anemia requiring chronic transfusion therapy; (2) assess the extent of iron overload and patterns of care surrounding the use of iron chelation therapy in different geographical regions.
Methods
Patients aged >2 years requiring chronic transfusion therapy with newly diagnosed anemias (<12 months from diagnosis), including thalassemia, low and intermediate-1 myelodysplastic syndromes (MDS), aplastic anemia (AA), Diamond-Blackfan anemia (DBA) and other transfusion-dependent anemias were enrolled. Patients were recruited from Turkey, Russia and South Africa as well as from countries within the Asia-Pacific and Middle East regions. Patients with secondary or therapy-related MDS; intermediate-2 or high-risk MDS; or acute leukemia were excluded. Patients were evaluated at baseline and at follow-up visits according to the standard practice for up to 3 years or until death. At baseline, patient demographics and details of iron overload and iron chelation therapy use were assessed. Transfusional hemosiderosis was defined as ≥1 serum ferritin measurement >1000 ng/mL after onset of transfusion therapy; or a liver iron concentration (LIC) >2 mg Fe/g dry weight; or if serum ferritin or LIC measurements were not available, evidence of ≥20 units of red blood cell transfusions.
Results
Of the 564 patients (including patients aged ≤18 years, n=57) recruited, majority had a diagnosis of MDS (58.5%, n=330), followed by AA (31.2%, n=176), other transfusion-dependent disorders (6.2%, n=35), β-thalassemia major (2.0%, n=11), β-thalassemia intermedia (1.1%, n=6), sickle cell anemia (0.5%, n=3) and DBA (0.4%, n=2). The mean age (±SD) of the patients was 51.9±23.87 years (range, 2-92); 49.5% (n=279) were male. At study entry, >70% of patients in all countries, except Thailand (45.7%), were receiving transfusion therapy. Overall, 29.4% (166/564) patients and 36.8% (21/57) pediatric patients were assessed as having iron overload during the study; extent of iron overload across countries varied and ranged from 7.4% (6/81) in Thailand to 83.3% (10/12) in Hong Kong. Patients receiving drug therapy only were less likely to be assessed for iron overload (1.2% [1/86]) than patients receiving erythrocyte transfusion (33.0% [33/100]) or erythrocyte transfusion and drug therapy (38.1% [131/344]). Overall, 39% of the population received iron chelation therapy and ranged from 9.2% (11/119) in China to 100.0% (12/12) in Hong Kong. Despite receiving iron chelation therapy, 56.4% (124/220 patients) were documented as having iron overload (Figure 1).
Summary
In this large observational study, MDS was the most common disease type enrolled. Majority of patients received transfusion therapy across countries; consequently there was a proportion of patients that had iron overload. Despite a large percent of patients receiving transfusion therapy and having developed iron overload, there was a broad spectrum of iron chelation use. Notably, over half of patients still had iron overload despite receiving chelation therapy. Overall, these results suggest that iron overload management practices may still be suboptimal in many parts of the world. Further studies are required to clearly understand these geographical differences in management of transfusion–dependent anemias.
Keyword(s): Clinical data, Iron chelation, Iron overload, Transfusion

Session topic: Publication Only
{{ help_message }}
{{filter}}


