Hematologia

Contributions
Type: Publication Only
Background
There is considerable evidence that pediatric (PED), high intensity chemotherapy protocols may improve clinical outcome in adolescents and young adults (AYA) with Acute Lymphoblastic Leukemia (ALL). Toxicity, which is more frequent and severe in older patients (pts), is a key issue, limiting the potential benefits and becoming more evident outside clinical trials.
Aims
We evaluated the feasibility and safety of a PED regimen – the Dana-Farber Cancer Institute Consortium Protocol (DFCI) 05-01 - in Ph- AYA ALL pts receiving treatment (Tx) in an adult Hematology reference center.
Methods
We conducted a retrospective analysis of all adverse events (AE) occurring during the DFCI 05-01 regimen in a series of consecutive AYA pts treated at our institution, with a focus on Grade 3-5 CTCAE toxicities and special interest side effects with at least a possible relationship to Tx.
Results
22 Ph- ALL pts (median age 18 yo, range 15-42, 68.2% males), diagnosed between 2007 and 2014, were treated according to the DFCI 05-01 protocol. 54.6% were <20 yo, 13.6% 20-29 yo, and 31.8% were 30-42 yo. B-ALL was diagnosed in 11 (50%), T-ALL in 8 (36.4%), and T Lymphoblastic Lymphoma in 3 (13.6%). Only 1 case had detectable blast cells in diagnostic spinal tap (<5/ul). At the end of induction (IND) 20 (91%) pts achieved CR, 2 had refractory disease, being excluded from the analysis after this point, and 4 B-ALL cases had high MRD levels (>0.001) assessed by RQ-PCR. Based on cytogenetics and molecular response at day 32, 7 (31.8%) pts moved to the very high-risk group and received intensified Tx according to protocol. 2 relapses occurred before maintenance and 2 pts died of refractory disease shortly after starting salvage regimens.
2 Tx-related deaths were observed. With a median follow up of 37 months, 16 pts (72.7%) are alive in CR1: 5 completed the protocol, 8 had an allogeneic stem-cell transplant after Consolidation II, and 3 are still under Tx.
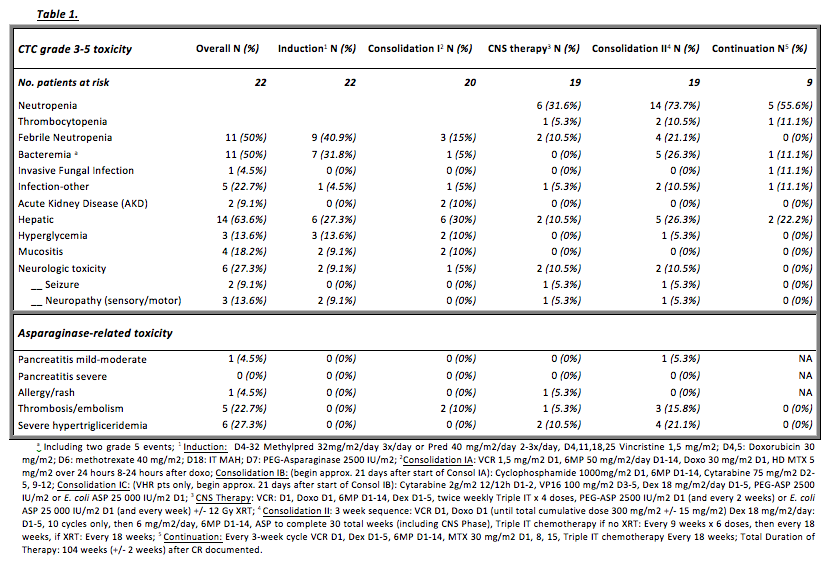
TABLE 1 depicts the grade 3-5 toxicities and asparaginase (ASP) specific related toxicities observed during a median of 11 months of Tx per pt.
No deaths were observed during IND and infectious complications were the most frequent AE: 9 pts (40.9%) developed febrile neutropenia with 7 confirmed bacteremias. Overall, infections were the most relevant reported AE during Tx, with 2 grade 5 events. 2 episodes of reversible AKD (not requiring renal-replacement therapy) during high dose MTX Tx were seen. 10/14 evaluable pts (71.4%) who initiated the 30-week ASP course were able to receive 26 or more IM E. coli ASP equivalent doses, with one of the ASP formulations – native E. coli ASP, PEG-L-ASP, or Erwinia L-ASP. 4 musculoskeletal AEs were observed, including 3 cases of avascular necrosis, and 1 bone fracture, possibly related to corticosteroid therapy.
Summary
Increased toxicity in AYA is the limiting factor for the use of intensified therapeutic regimens in ALL. We did not observe unexpected grade 3-4 AE, nor an unacceptable high incidence of these events, compared to literature. Tx-related mortality was also equivalent (9%). In this series ASP tolerance was acceptable: the incidence of major ASP-related toxicities was similar to older children, with a proportion of pts completing at least 26 weeks of high-dose ASP only slightly lower than described in PED populations (71.4% VS 88%). The application of tolerable, PED protocols may improve the outcome of adult ALL.
Keyword(s): Acute lymphoblastic leukemia, Adolescents, Toxicity, Young adult
Session topic: Publication Only
Type: Publication Only
Background
There is considerable evidence that pediatric (PED), high intensity chemotherapy protocols may improve clinical outcome in adolescents and young adults (AYA) with Acute Lymphoblastic Leukemia (ALL). Toxicity, which is more frequent and severe in older patients (pts), is a key issue, limiting the potential benefits and becoming more evident outside clinical trials.
Aims
We evaluated the feasibility and safety of a PED regimen – the Dana-Farber Cancer Institute Consortium Protocol (DFCI) 05-01 - in Ph- AYA ALL pts receiving treatment (Tx) in an adult Hematology reference center.
Methods
We conducted a retrospective analysis of all adverse events (AE) occurring during the DFCI 05-01 regimen in a series of consecutive AYA pts treated at our institution, with a focus on Grade 3-5 CTCAE toxicities and special interest side effects with at least a possible relationship to Tx.
Results
22 Ph- ALL pts (median age 18 yo, range 15-42, 68.2% males), diagnosed between 2007 and 2014, were treated according to the DFCI 05-01 protocol. 54.6% were <20 yo, 13.6% 20-29 yo, and 31.8% were 30-42 yo. B-ALL was diagnosed in 11 (50%), T-ALL in 8 (36.4%), and T Lymphoblastic Lymphoma in 3 (13.6%). Only 1 case had detectable blast cells in diagnostic spinal tap (<5/ul). At the end of induction (IND) 20 (91%) pts achieved CR, 2 had refractory disease, being excluded from the analysis after this point, and 4 B-ALL cases had high MRD levels (>0.001) assessed by RQ-PCR. Based on cytogenetics and molecular response at day 32, 7 (31.8%) pts moved to the very high-risk group and received intensified Tx according to protocol. 2 relapses occurred before maintenance and 2 pts died of refractory disease shortly after starting salvage regimens.
2 Tx-related deaths were observed. With a median follow up of 37 months, 16 pts (72.7%) are alive in CR1: 5 completed the protocol, 8 had an allogeneic stem-cell transplant after Consolidation II, and 3 are still under Tx.
TABLE 1 depicts the grade 3-5 toxicities and asparaginase (ASP) specific related toxicities observed during a median of 11 months of Tx per pt.
No deaths were observed during IND and infectious complications were the most frequent AE: 9 pts (40.9%) developed febrile neutropenia with 7 confirmed bacteremias. Overall, infections were the most relevant reported AE during Tx, with 2 grade 5 events. 2 episodes of reversible AKD (not requiring renal-replacement therapy) during high dose MTX Tx were seen. 10/14 evaluable pts (71.4%) who initiated the 30-week ASP course were able to receive 26 or more IM E. coli ASP equivalent doses, with one of the ASP formulations – native E. coli ASP, PEG-L-ASP, or Erwinia L-ASP. 4 musculoskeletal AEs were observed, including 3 cases of avascular necrosis, and 1 bone fracture, possibly related to corticosteroid therapy.
Summary
Increased toxicity in AYA is the limiting factor for the use of intensified therapeutic regimens in ALL. We did not observe unexpected grade 3-4 AE, nor an unacceptable high incidence of these events, compared to literature. Tx-related mortality was also equivalent (9%). In this series ASP tolerance was acceptable: the incidence of major ASP-related toxicities was similar to older children, with a proportion of pts completing at least 26 weeks of high-dose ASP only slightly lower than described in PED populations (71.4% VS 88%). The application of tolerable, PED protocols may improve the outcome of adult ALL.
Keyword(s): Acute lymphoblastic leukemia, Adolescents, Toxicity, Young adult

Session topic: Publication Only


