THE MDS/MPN PATIENTS TREATED WITH 5-AZACITIDINE: A CLINICAL REAL LIFE EXPERIENCE
(Abstract release date: 05/21/15)
EHA Library. Riva M. 06/12/15; 102716; PB1833
Disclosure(s): AO Niguarda Ca' Granda MilanHematology

Marta Riva
Contributions
Contributions
Abstract
Abstract: PB1833
Type: Publication Only
Background
The myelodysplastic/myeloproliferative disorders (MDS/MPN), according to the World Health Organization classification, include cases that have clinical, laboratory, and morphologic features intermediate between myelodysplastic syndrome (MDS) and myeloproliferative neoplasm (MPN). This is an infrequent entity and there is few data in the literature regarding treatment.
Aims
To evaluate the efficacy of 5-azacitidine (5-AZA) treatment in patients (pts) affected by MDS/MPN disorders.
Methods
A retrospective analysis of the MDS/MPN pts, into a large cohort of MDS or AML with blast < 30% subjects, treated with 5-AZA in 10 centers in Lombardia region was performed. Response to therapy was considered evaluable if pts had reached, at the time of observation, at least 6 cycles of 5-AZA. The Kaplan Meier method was applied to evaluate the survival.
Results
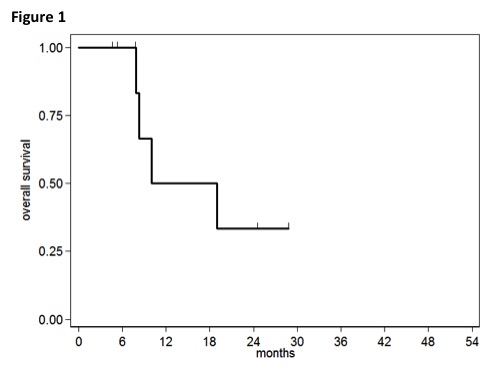
From a population of 187 pts, data about 9 cases of MDS/MPN were carried out. Of these 4 were female and 5 male. Median age was 65 (range 23-81). Median bone marrow blasts was 5% (range 2-15); in two cases blasts were over 10%. In 3 pts Red Blood Cells transfusion dependence was present. In the other 6 cases median hemoglobin level was 10.2 g/dl (range 9-15). Only 1 patient had Platelet count (PLT) < 10.000/mm3, in 3 case PLT was < 50.000/mm3 and in 2 cases PLT was > 300.000/mm3. In 3 pts the Neutrophil count was ≥ 10.000/mm3, in 1 case was < 1500/mm3. The median number of 5-AZA courses administered was 6 (range 5-20). A response to the treatment (including the cases of “stable disease” maintenance) was achieved in 5 cases on 7 evaluable. The median survival was 10 months (Figure 1).
Summary
This abstract is intended to be only a descriptive report of a disease entity rarely considered. The small sample size does not allow to draw any conclusion about the observed population. Nevertheless, the response rate to 5-AZA therapy seems to indicate a trend of efficacy and, therefore, the interest in collecting new data to make a significant analysis.
Type: Publication Only
Background
The myelodysplastic/myeloproliferative disorders (MDS/MPN), according to the World Health Organization classification, include cases that have clinical, laboratory, and morphologic features intermediate between myelodysplastic syndrome (MDS) and myeloproliferative neoplasm (MPN). This is an infrequent entity and there is few data in the literature regarding treatment.
Aims
To evaluate the efficacy of 5-azacitidine (5-AZA) treatment in patients (pts) affected by MDS/MPN disorders.
Methods
A retrospective analysis of the MDS/MPN pts, into a large cohort of MDS or AML with blast < 30% subjects, treated with 5-AZA in 10 centers in Lombardia region was performed. Response to therapy was considered evaluable if pts had reached, at the time of observation, at least 6 cycles of 5-AZA. The Kaplan Meier method was applied to evaluate the survival.
Results
From a population of 187 pts, data about 9 cases of MDS/MPN were carried out. Of these 4 were female and 5 male. Median age was 65 (range 23-81). Median bone marrow blasts was 5% (range 2-15); in two cases blasts were over 10%. In 3 pts Red Blood Cells transfusion dependence was present. In the other 6 cases median hemoglobin level was 10.2 g/dl (range 9-15). Only 1 patient had Platelet count (PLT) < 10.000/mm3, in 3 case PLT was < 50.000/mm3 and in 2 cases PLT was > 300.000/mm3. In 3 pts the Neutrophil count was ≥ 10.000/mm3, in 1 case was < 1500/mm3. The median number of 5-AZA courses administered was 6 (range 5-20). A response to the treatment (including the cases of “stable disease” maintenance) was achieved in 5 cases on 7 evaluable. The median survival was 10 months (Figure 1).
Summary
This abstract is intended to be only a descriptive report of a disease entity rarely considered. The small sample size does not allow to draw any conclusion about the observed population. Nevertheless, the response rate to 5-AZA therapy seems to indicate a trend of efficacy and, therefore, the interest in collecting new data to make a significant analysis.

Abstract: PB1833
Type: Publication Only
Background
The myelodysplastic/myeloproliferative disorders (MDS/MPN), according to the World Health Organization classification, include cases that have clinical, laboratory, and morphologic features intermediate between myelodysplastic syndrome (MDS) and myeloproliferative neoplasm (MPN). This is an infrequent entity and there is few data in the literature regarding treatment.
Aims
To evaluate the efficacy of 5-azacitidine (5-AZA) treatment in patients (pts) affected by MDS/MPN disorders.
Methods
A retrospective analysis of the MDS/MPN pts, into a large cohort of MDS or AML with blast < 30% subjects, treated with 5-AZA in 10 centers in Lombardia region was performed. Response to therapy was considered evaluable if pts had reached, at the time of observation, at least 6 cycles of 5-AZA. The Kaplan Meier method was applied to evaluate the survival.
Results
From a population of 187 pts, data about 9 cases of MDS/MPN were carried out. Of these 4 were female and 5 male. Median age was 65 (range 23-81). Median bone marrow blasts was 5% (range 2-15); in two cases blasts were over 10%. In 3 pts Red Blood Cells transfusion dependence was present. In the other 6 cases median hemoglobin level was 10.2 g/dl (range 9-15). Only 1 patient had Platelet count (PLT) < 10.000/mm3, in 3 case PLT was < 50.000/mm3 and in 2 cases PLT was > 300.000/mm3. In 3 pts the Neutrophil count was ≥ 10.000/mm3, in 1 case was < 1500/mm3. The median number of 5-AZA courses administered was 6 (range 5-20). A response to the treatment (including the cases of “stable disease” maintenance) was achieved in 5 cases on 7 evaluable. The median survival was 10 months (Figure 1).
Summary
This abstract is intended to be only a descriptive report of a disease entity rarely considered. The small sample size does not allow to draw any conclusion about the observed population. Nevertheless, the response rate to 5-AZA therapy seems to indicate a trend of efficacy and, therefore, the interest in collecting new data to make a significant analysis.

Type: Publication Only
Background
The myelodysplastic/myeloproliferative disorders (MDS/MPN), according to the World Health Organization classification, include cases that have clinical, laboratory, and morphologic features intermediate between myelodysplastic syndrome (MDS) and myeloproliferative neoplasm (MPN). This is an infrequent entity and there is few data in the literature regarding treatment.
Aims
To evaluate the efficacy of 5-azacitidine (5-AZA) treatment in patients (pts) affected by MDS/MPN disorders.
Methods
A retrospective analysis of the MDS/MPN pts, into a large cohort of MDS or AML with blast < 30% subjects, treated with 5-AZA in 10 centers in Lombardia region was performed. Response to therapy was considered evaluable if pts had reached, at the time of observation, at least 6 cycles of 5-AZA. The Kaplan Meier method was applied to evaluate the survival.
Results
From a population of 187 pts, data about 9 cases of MDS/MPN were carried out. Of these 4 were female and 5 male. Median age was 65 (range 23-81). Median bone marrow blasts was 5% (range 2-15); in two cases blasts were over 10%. In 3 pts Red Blood Cells transfusion dependence was present. In the other 6 cases median hemoglobin level was 10.2 g/dl (range 9-15). Only 1 patient had Platelet count (PLT) < 10.000/mm3, in 3 case PLT was < 50.000/mm3 and in 2 cases PLT was > 300.000/mm3. In 3 pts the Neutrophil count was ≥ 10.000/mm3, in 1 case was < 1500/mm3. The median number of 5-AZA courses administered was 6 (range 5-20). A response to the treatment (including the cases of “stable disease” maintenance) was achieved in 5 cases on 7 evaluable. The median survival was 10 months (Figure 1).
Summary
This abstract is intended to be only a descriptive report of a disease entity rarely considered. The small sample size does not allow to draw any conclusion about the observed population. Nevertheless, the response rate to 5-AZA therapy seems to indicate a trend of efficacy and, therefore, the interest in collecting new data to make a significant analysis.

{{ help_message }}
{{filter}}


