HEMATOLOGY

Contributions
Type: Publication Only
Background
Tyrosine kinase inhibitors have dramatically changed the treatment of CML and nowadays most of the patients are able to achive sustained deep molecular responses in this setting. This scenario has led to an increasing interest on therapy discontinuation studies but this strategy is not recommended outside clinical trials
Aims
We have reduced the dose of Imatinib (IM) from the standard 400 to 300 mg/day, in some patients with deep and stable deep responses in order to improve tolerability and costs of the treatment. In this analysis we review whether this strategy was safe and clinically useful.
Methods
Patients with chronic phase CML that decreased IM 400 to 300 mg/day at least during 3 months and who had molecular responses analyzed before and after the change were retrospectively selected from our database. BCR-ABL ratio was measured by automated means (GeneXpert, Cepheid, CA, USA) according to the International-Scale. Hematological parameters such as hemoglobin level and mean corpuscular volume were also analyzed before and after the IM dose change. Data were analyzed using SPSS v11.0.
Results
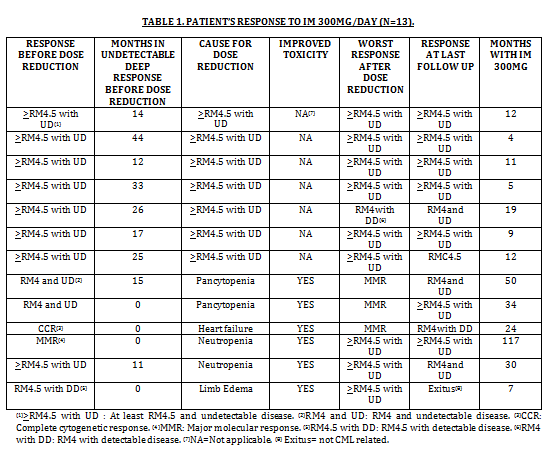
A total of 13 patients (7 males, 6 females) with chronic-phase CML diagnosed between 1998 and 2012 were included in the present study. Median age at diagnosis was 67 years (range 23-78). According to Sokal score, 3 were high risk, 4 intermediate and 6 low; according to EURO score, 1 was high risk, 6 intermediate and 6 low; according to EUTOS score, all were low risk. First line treatment was IM 400 mg in 10 (76.9%), IM 600 mg in 1 (7.1%) and Interferon-alpha in 2 (16%). The reason for dose reduction was toxicity in 6 patients (46%): neutropenia (2), pancytopenia (2), limb edema (1) and heart failure (1). In all of them the adverse event improved after lowering the dose. All patients that reduced the dose due to toxicity were at least in CCR. In the other 7 patients (54%) the dose was reduced because of sustained deep response; RM4.5 or better and undetectable disease (>RM4.5 with UD). Median time in >RM4.5 with UD, prior to dose reduction was 24.4 months (range 12-33; Table 1). Median time of treatment with 300 mg/day was 12 months (range 4-117). Comparing molecular response before dose reduction and at last follow up, 3 patients improved and 10 maintained it; if we compared molecular response before the dose reduction and the worst response during follow up, just 3 patients worsened (2 from RM4 and UD to MMR, and 1 from RM4.5 and UD to RM4 with detectable disease), but none of this changes were clinically relevant. The median hemoglobin increase was 1.2 g/dl (range 0.4-2.3; p=0,054). The median decrease in mean corpuscular volume was 3.7 fL (range 0.4-11.5; p=0,006) (Wilcoxon Test). With this univariate analysis we cannot rule out that other concurrent causes could contribute to this hematological change. Additionally, there was an economical benefit derived from the dose reduction, since 334 accumulated months of treatment with IM 300 mg/day, produced savings of 357.046 Euros (savings for 13 patients would be of 166.764 Euros per year). Out of the 13 patients, 12 continue with 300 mg, and one patient died due to causes not related to CML
Summary
In our experience IM dose reduction due to medical decision or toxicity in patients with sustained deep response has been safe, clinically useful and economically beneficial. This could be a useful strategy in some patients with deep responses until treatment discontinuation could be recommended in common clinical practice.
Keyword(s): Chronic myeloid leukemia, Imatinib, Molecular response, Toxicity
Type: Publication Only
Background
Tyrosine kinase inhibitors have dramatically changed the treatment of CML and nowadays most of the patients are able to achive sustained deep molecular responses in this setting. This scenario has led to an increasing interest on therapy discontinuation studies but this strategy is not recommended outside clinical trials
Aims
We have reduced the dose of Imatinib (IM) from the standard 400 to 300 mg/day, in some patients with deep and stable deep responses in order to improve tolerability and costs of the treatment. In this analysis we review whether this strategy was safe and clinically useful.
Methods
Patients with chronic phase CML that decreased IM 400 to 300 mg/day at least during 3 months and who had molecular responses analyzed before and after the change were retrospectively selected from our database. BCR-ABL ratio was measured by automated means (GeneXpert, Cepheid, CA, USA) according to the International-Scale. Hematological parameters such as hemoglobin level and mean corpuscular volume were also analyzed before and after the IM dose change. Data were analyzed using SPSS v11.0.
Results
A total of 13 patients (7 males, 6 females) with chronic-phase CML diagnosed between 1998 and 2012 were included in the present study. Median age at diagnosis was 67 years (range 23-78). According to Sokal score, 3 were high risk, 4 intermediate and 6 low; according to EURO score, 1 was high risk, 6 intermediate and 6 low; according to EUTOS score, all were low risk. First line treatment was IM 400 mg in 10 (76.9%), IM 600 mg in 1 (7.1%) and Interferon-alpha in 2 (16%). The reason for dose reduction was toxicity in 6 patients (46%): neutropenia (2), pancytopenia (2), limb edema (1) and heart failure (1). In all of them the adverse event improved after lowering the dose. All patients that reduced the dose due to toxicity were at least in CCR. In the other 7 patients (54%) the dose was reduced because of sustained deep response; RM4.5 or better and undetectable disease (>RM4.5 with UD). Median time in >RM4.5 with UD, prior to dose reduction was 24.4 months (range 12-33; Table 1). Median time of treatment with 300 mg/day was 12 months (range 4-117). Comparing molecular response before dose reduction and at last follow up, 3 patients improved and 10 maintained it; if we compared molecular response before the dose reduction and the worst response during follow up, just 3 patients worsened (2 from RM4 and UD to MMR, and 1 from RM4.5 and UD to RM4 with detectable disease), but none of this changes were clinically relevant. The median hemoglobin increase was 1.2 g/dl (range 0.4-2.3; p=0,054). The median decrease in mean corpuscular volume was 3.7 fL (range 0.4-11.5; p=0,006) (Wilcoxon Test). With this univariate analysis we cannot rule out that other concurrent causes could contribute to this hematological change. Additionally, there was an economical benefit derived from the dose reduction, since 334 accumulated months of treatment with IM 300 mg/day, produced savings of 357.046 Euros (savings for 13 patients would be of 166.764 Euros per year). Out of the 13 patients, 12 continue with 300 mg, and one patient died due to causes not related to CML
Summary
In our experience IM dose reduction due to medical decision or toxicity in patients with sustained deep response has been safe, clinically useful and economically beneficial. This could be a useful strategy in some patients with deep responses until treatment discontinuation could be recommended in common clinical practice.
Keyword(s): Chronic myeloid leukemia, Imatinib, Molecular response, Toxicity



