CORRELATION OF DASATINIB PHARMACOKINETICS WITH CLINICAL RESPONSE AND ADVERSE EVENTS IN NEWLY DIAGNOSED CHRONIC MYELOID LEUKEMIA CHRONIC PHASE
(Abstract release date: 05/21/15)
EHA Library. Mita A. 06/12/15; 102688; PB1750
Disclosure(s): Akita university
Akiko Mita
Contributions
Contributions
Abstract
Abstract: PB1750
Type: Publication Only
Background
The second-generation tyrosine kinase inhibitor (TKI) dasatinib is used as the first-line therapy for newly diagnosed chronic myeloid leukemia (CML). However, some patients fail to respond or become intolerant to dasatinib. Several studies have shown a relationship between pharmacokinetics of dasatinib and molecular responses or significant adverse events.
Aims
To investigate pharmacokinetics of dasatinib and to identify an influence of transporter gene polymorphisms on dasatinib treatment in newly diagnosed CML patients.
Methods
Fourteen patients treated with dasatinib from whom were obtained a total of 118 pharmacokinetic profiles were enrolled in this study. Dasatinib plasma concentrations from samples obtained just prior to and 1, 2, and 4 h after oral dasatinib administration were analyzed by liquid chromatography-tandem mass spectrometry. Genotyping of 10 single nucleotide polymorphisms (SNPs) in genes involved in dasatinib pharmacokinetics (ABCG2, ABCB1, CYP3A5*3, SLC22A1, ABCC2) was performed by PCR-RFLP. The study protocol was approved by the Ethics Committee of Akita University Hospital, and all recipients gave written informed consent.
Results
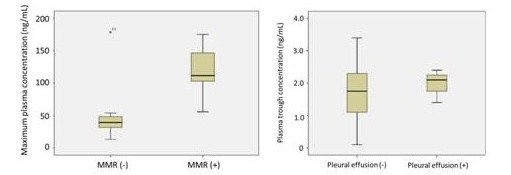
In this study, associations between dasatinib concentration, clinical response, adverse event, and 10 SNPs were investigated among newly diagnosed chronic phase CML patients. Seven patients achieved major molecular response (MMR) at 3 month and the other 7 patients did not. The plasma concentration at 2 h (C2h) or the maximum plasma concentration (Cmax) of dasatinib was significantly higher among patients with a MMR at 3 month than those without (P=0.051, P=0.035, Figure 1). Among 118 pharmacokinetics profiles, there were 8 adverse events of pleural effusion in 4 patients. Although there was not a significant difference in plasma trough concentration of dasatinib (C0h) between groups with pleural effusion and without (P=0.350), it was observed in patients with more than 1.4 ng/mL of C0h which could be translated into 2.8 nM (Figure 2). 5 patients have ABCG2 421C/C and 9 patients have ABCG2 421C/A. There were significant differences of plasma concentration at 2 h (C2h) after dasatinib administration between two groups (P=0.019). However, the other SNPs were not associated with dasatinib pharmacokinetics in this study.
Summary
Higher dasatinib C2h/max was associated with the likelihood of achieving a MMR at 3 month in our small CML patient cohort. Additionally, pleural effusion was not observed in patients with lower dasatinib trough concentration. Although a prospective study with a larger patient population is necessary to validate these findings, in addition to BCR-ABL1 mutation analysis, our data indicate that clinical dasatinib blood-level testing may improve the efficacy and the safety of dasatinib therapy among newly diagnosed CML patients.
Keyword(s): Chronic myeloid leukemia, Gene polymorphism, Pharmacokinetic, Tyrosine kinase inhibitor
Type: Publication Only
Background
The second-generation tyrosine kinase inhibitor (TKI) dasatinib is used as the first-line therapy for newly diagnosed chronic myeloid leukemia (CML). However, some patients fail to respond or become intolerant to dasatinib. Several studies have shown a relationship between pharmacokinetics of dasatinib and molecular responses or significant adverse events.
Aims
To investigate pharmacokinetics of dasatinib and to identify an influence of transporter gene polymorphisms on dasatinib treatment in newly diagnosed CML patients.
Methods
Fourteen patients treated with dasatinib from whom were obtained a total of 118 pharmacokinetic profiles were enrolled in this study. Dasatinib plasma concentrations from samples obtained just prior to and 1, 2, and 4 h after oral dasatinib administration were analyzed by liquid chromatography-tandem mass spectrometry. Genotyping of 10 single nucleotide polymorphisms (SNPs) in genes involved in dasatinib pharmacokinetics (ABCG2, ABCB1, CYP3A5*3, SLC22A1, ABCC2) was performed by PCR-RFLP. The study protocol was approved by the Ethics Committee of Akita University Hospital, and all recipients gave written informed consent.
Results
In this study, associations between dasatinib concentration, clinical response, adverse event, and 10 SNPs were investigated among newly diagnosed chronic phase CML patients. Seven patients achieved major molecular response (MMR) at 3 month and the other 7 patients did not. The plasma concentration at 2 h (C2h) or the maximum plasma concentration (Cmax) of dasatinib was significantly higher among patients with a MMR at 3 month than those without (P=0.051, P=0.035, Figure 1). Among 118 pharmacokinetics profiles, there were 8 adverse events of pleural effusion in 4 patients. Although there was not a significant difference in plasma trough concentration of dasatinib (C0h) between groups with pleural effusion and without (P=0.350), it was observed in patients with more than 1.4 ng/mL of C0h which could be translated into 2.8 nM (Figure 2). 5 patients have ABCG2 421C/C and 9 patients have ABCG2 421C/A. There were significant differences of plasma concentration at 2 h (C2h) after dasatinib administration between two groups (P=0.019). However, the other SNPs were not associated with dasatinib pharmacokinetics in this study.
Summary
Higher dasatinib C2h/max was associated with the likelihood of achieving a MMR at 3 month in our small CML patient cohort. Additionally, pleural effusion was not observed in patients with lower dasatinib trough concentration. Although a prospective study with a larger patient population is necessary to validate these findings, in addition to BCR-ABL1 mutation analysis, our data indicate that clinical dasatinib blood-level testing may improve the efficacy and the safety of dasatinib therapy among newly diagnosed CML patients.
Keyword(s): Chronic myeloid leukemia, Gene polymorphism, Pharmacokinetic, Tyrosine kinase inhibitor

Abstract: PB1750
Type: Publication Only
Background
The second-generation tyrosine kinase inhibitor (TKI) dasatinib is used as the first-line therapy for newly diagnosed chronic myeloid leukemia (CML). However, some patients fail to respond or become intolerant to dasatinib. Several studies have shown a relationship between pharmacokinetics of dasatinib and molecular responses or significant adverse events.
Aims
To investigate pharmacokinetics of dasatinib and to identify an influence of transporter gene polymorphisms on dasatinib treatment in newly diagnosed CML patients.
Methods
Fourteen patients treated with dasatinib from whom were obtained a total of 118 pharmacokinetic profiles were enrolled in this study. Dasatinib plasma concentrations from samples obtained just prior to and 1, 2, and 4 h after oral dasatinib administration were analyzed by liquid chromatography-tandem mass spectrometry. Genotyping of 10 single nucleotide polymorphisms (SNPs) in genes involved in dasatinib pharmacokinetics (ABCG2, ABCB1, CYP3A5*3, SLC22A1, ABCC2) was performed by PCR-RFLP. The study protocol was approved by the Ethics Committee of Akita University Hospital, and all recipients gave written informed consent.
Results
In this study, associations between dasatinib concentration, clinical response, adverse event, and 10 SNPs were investigated among newly diagnosed chronic phase CML patients. Seven patients achieved major molecular response (MMR) at 3 month and the other 7 patients did not. The plasma concentration at 2 h (C2h) or the maximum plasma concentration (Cmax) of dasatinib was significantly higher among patients with a MMR at 3 month than those without (P=0.051, P=0.035, Figure 1). Among 118 pharmacokinetics profiles, there were 8 adverse events of pleural effusion in 4 patients. Although there was not a significant difference in plasma trough concentration of dasatinib (C0h) between groups with pleural effusion and without (P=0.350), it was observed in patients with more than 1.4 ng/mL of C0h which could be translated into 2.8 nM (Figure 2). 5 patients have ABCG2 421C/C and 9 patients have ABCG2 421C/A. There were significant differences of plasma concentration at 2 h (C2h) after dasatinib administration between two groups (P=0.019). However, the other SNPs were not associated with dasatinib pharmacokinetics in this study.
Summary
Higher dasatinib C2h/max was associated with the likelihood of achieving a MMR at 3 month in our small CML patient cohort. Additionally, pleural effusion was not observed in patients with lower dasatinib trough concentration. Although a prospective study with a larger patient population is necessary to validate these findings, in addition to BCR-ABL1 mutation analysis, our data indicate that clinical dasatinib blood-level testing may improve the efficacy and the safety of dasatinib therapy among newly diagnosed CML patients.
Keyword(s): Chronic myeloid leukemia, Gene polymorphism, Pharmacokinetic, Tyrosine kinase inhibitor

Type: Publication Only
Background
The second-generation tyrosine kinase inhibitor (TKI) dasatinib is used as the first-line therapy for newly diagnosed chronic myeloid leukemia (CML). However, some patients fail to respond or become intolerant to dasatinib. Several studies have shown a relationship between pharmacokinetics of dasatinib and molecular responses or significant adverse events.
Aims
To investigate pharmacokinetics of dasatinib and to identify an influence of transporter gene polymorphisms on dasatinib treatment in newly diagnosed CML patients.
Methods
Fourteen patients treated with dasatinib from whom were obtained a total of 118 pharmacokinetic profiles were enrolled in this study. Dasatinib plasma concentrations from samples obtained just prior to and 1, 2, and 4 h after oral dasatinib administration were analyzed by liquid chromatography-tandem mass spectrometry. Genotyping of 10 single nucleotide polymorphisms (SNPs) in genes involved in dasatinib pharmacokinetics (ABCG2, ABCB1, CYP3A5*3, SLC22A1, ABCC2) was performed by PCR-RFLP. The study protocol was approved by the Ethics Committee of Akita University Hospital, and all recipients gave written informed consent.
Results
In this study, associations between dasatinib concentration, clinical response, adverse event, and 10 SNPs were investigated among newly diagnosed chronic phase CML patients. Seven patients achieved major molecular response (MMR) at 3 month and the other 7 patients did not. The plasma concentration at 2 h (C2h) or the maximum plasma concentration (Cmax) of dasatinib was significantly higher among patients with a MMR at 3 month than those without (P=0.051, P=0.035, Figure 1). Among 118 pharmacokinetics profiles, there were 8 adverse events of pleural effusion in 4 patients. Although there was not a significant difference in plasma trough concentration of dasatinib (C0h) between groups with pleural effusion and without (P=0.350), it was observed in patients with more than 1.4 ng/mL of C0h which could be translated into 2.8 nM (Figure 2). 5 patients have ABCG2 421C/C and 9 patients have ABCG2 421C/A. There were significant differences of plasma concentration at 2 h (C2h) after dasatinib administration between two groups (P=0.019). However, the other SNPs were not associated with dasatinib pharmacokinetics in this study.
Summary
Higher dasatinib C2h/max was associated with the likelihood of achieving a MMR at 3 month in our small CML patient cohort. Additionally, pleural effusion was not observed in patients with lower dasatinib trough concentration. Although a prospective study with a larger patient population is necessary to validate these findings, in addition to BCR-ABL1 mutation analysis, our data indicate that clinical dasatinib blood-level testing may improve the efficacy and the safety of dasatinib therapy among newly diagnosed CML patients.
Keyword(s): Chronic myeloid leukemia, Gene polymorphism, Pharmacokinetic, Tyrosine kinase inhibitor

{{ help_message }}
{{filter}}


