Hematology & BMT Unit, Hospital de Especialidades, CMN 'La Raza'

Contributions
Type: Publication Only
Background
Long term prognosis of patients with chronic myeloid leukemia (CML) has significantly improved during the “age of tyrosine kinase inhibitors”, though the occurrence of adverse events on medium and long term in patients with chronic comorbidities, of which cardiopulmonary and metabolic diseases must be routinely evaluated, has become a clinical concern albeit the cardiac safety profile has been prospectively investigated. During the phase II and III clinical trials of both first and second-line therapy, we reported our experience in cardiologic monitoring of patients with CML treated with second-generation TKI (nilotinib and dasatinib) on second-line therapy.
Aims
Identify clinical, electrocardiographic and transthoracic echocardiogram alterations in CML patients treated with nilotinib or dasatinib.
Methods
Clinical evaluation, electrocardiograms and transthoracic echocardiogram were performed to all CML patients on treatment with nilotinib or dasatinib during the period between October 2010 and January 2015, at the beginning of treatment and every 6 and 12 months after that according to the occurrence or not of abnormalities detected at baseline. The exclusion criteria were; clinically significant cardiac disease (congestive heart failure), left ventricular ejection fraction (LVEF) < 45%, past myocardial infarction or unstable angina (during the last 12 months), resting bradycardia (< 50 beats/min), left bundle branch block, ventricular bypass, acquired or congenital long QT syndrome (including family history), QTcF interval > 450 msec, patients taking medications that could extend the QT interval and those who could not discontinue their treatment before initiating nilotinib or dasatinib. The doses used of tyrosine kinase inhibitors were 400 mg every 12 hours for nilotinib and 100 mg every 24 hours for dasatinib per os.
Results
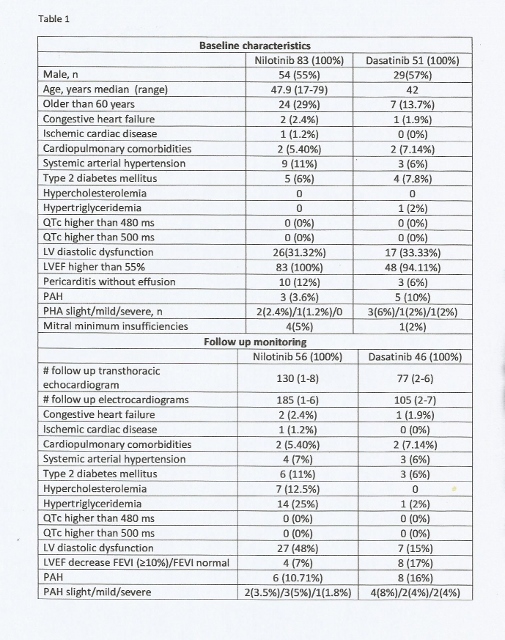
Baseline characteristics and electrocardiogram and transthoracic echocardiogram abnormalities are shown on table 1, along with the identified changes in the monitoring during treatment of the clinical, electrocardiogram and transthoracic abnormalities recorded.
Summary
The cardiac adverse events in patients with CML undergoing treatment with second-generation TKI are not common and may be related to factors independent from treatment like age, cardiopulmonary and metabolic comorbidities and pharmacological interactions. Treatment was discontinued on those patients with recurrent PAH on dasatinib dose adjustment, though there was a temporary suspension in one nilotinib patient due a severe PAH, extended QT syndrome was not observed in any of the patients, however 8 patients undergoing treatment with dasatinib (16%) showed PAH (2 severe, 2 mild, and 4 slight), 5 were temporary suspensions and 2 resulted in definitive suspensions due severe PAH; In our experience the results do not differ significantly from the international publications. To our knowledge is the first report to adverse events in México for PAH secondary to second-generation TKIs.
Keyword(s): Chronic myeloid leukemia
Type: Publication Only
Background
Long term prognosis of patients with chronic myeloid leukemia (CML) has significantly improved during the “age of tyrosine kinase inhibitors”, though the occurrence of adverse events on medium and long term in patients with chronic comorbidities, of which cardiopulmonary and metabolic diseases must be routinely evaluated, has become a clinical concern albeit the cardiac safety profile has been prospectively investigated. During the phase II and III clinical trials of both first and second-line therapy, we reported our experience in cardiologic monitoring of patients with CML treated with second-generation TKI (nilotinib and dasatinib) on second-line therapy.
Aims
Identify clinical, electrocardiographic and transthoracic echocardiogram alterations in CML patients treated with nilotinib or dasatinib.
Methods
Clinical evaluation, electrocardiograms and transthoracic echocardiogram were performed to all CML patients on treatment with nilotinib or dasatinib during the period between October 2010 and January 2015, at the beginning of treatment and every 6 and 12 months after that according to the occurrence or not of abnormalities detected at baseline. The exclusion criteria were; clinically significant cardiac disease (congestive heart failure), left ventricular ejection fraction (LVEF) < 45%, past myocardial infarction or unstable angina (during the last 12 months), resting bradycardia (< 50 beats/min), left bundle branch block, ventricular bypass, acquired or congenital long QT syndrome (including family history), QTcF interval > 450 msec, patients taking medications that could extend the QT interval and those who could not discontinue their treatment before initiating nilotinib or dasatinib. The doses used of tyrosine kinase inhibitors were 400 mg every 12 hours for nilotinib and 100 mg every 24 hours for dasatinib per os.
Results
Baseline characteristics and electrocardiogram and transthoracic echocardiogram abnormalities are shown on table 1, along with the identified changes in the monitoring during treatment of the clinical, electrocardiogram and transthoracic abnormalities recorded.
Summary
The cardiac adverse events in patients with CML undergoing treatment with second-generation TKI are not common and may be related to factors independent from treatment like age, cardiopulmonary and metabolic comorbidities and pharmacological interactions. Treatment was discontinued on those patients with recurrent PAH on dasatinib dose adjustment, though there was a temporary suspension in one nilotinib patient due a severe PAH, extended QT syndrome was not observed in any of the patients, however 8 patients undergoing treatment with dasatinib (16%) showed PAH (2 severe, 2 mild, and 4 slight), 5 were temporary suspensions and 2 resulted in definitive suspensions due severe PAH; In our experience the results do not differ significantly from the international publications. To our knowledge is the first report to adverse events in México for PAH secondary to second-generation TKIs.
Keyword(s): Chronic myeloid leukemia



