
Contributions
Type: Publication Only
Background
AML with mutated NPM1 and its cell line OCI-AML3 carry the NPM1 mutation A and the heterozygous R882C mutation of the DNA (cytosine-5-)-4 methyltransferase 3 alpha (DNMT3A). The mutant DNMT3A protein exerts a dominant negative inhibition on the wild type protein resulting in focal hypomethylation. Of note, AML with mutated NPM1 harbors a distinct methylation profile among AML subtypes. S-adenosylmethionine (SAM) is a universal methyl donor and a coenzyme of DNMT3A. SAM has been used in the clinical practice as an antidepressant drug with very limited side effects. There are growing evidences of the antineoplastic activity of SAM in vitro and in murine models of solid cancers. Recently, the combination of SAM with demethylating agents showed an interesting in vitro synergistic cytotoxicity on breast cancer cells.
Aims
To explore the effect of SAM on OCI-AML3 cells and its dependence on DNMT3A activity.
Methods
We used the MTT assay to test the cytostatic effect of SAM iodide (Sigma-Aldrich, St. Louis -MO) on OCI-AML3 cells (DSMZ Leibniz Institut, Braunschweig -Germany). The cells were treated with SAM for 24h. Untreated and treated cells were tested in triplicate for each SAM concentration. Results were expressed as percentage of cells viability compared to the control (i.e. OCI-AML3 untreated cells). The Apoptosis assays were performed after 24h and 72h of SAM treatment using the Tali® Apoptosis Assay Kit–Annexin V Alexa Fluor®488/Propidium Iodide (Invitrogen, Carlsbad, CA) for cells staining, and the Tali Image Based Cytometer (Invitrogen) for cells counting. In order to verify if the observed effect was mediated by DNMT3A, we repeated the MTT assay after DNMT3A silencing with a predesigned SiRNA directed against DNMT3A (SiRNA ID HSS176224, life technologies). Transfection was performed on triplicates cultures with lipofectamine RNAiMax (Invitrogen) and transfection efficiency calculated by BLOCK-IT AlexaFluor Red Fluorescent Oligo (Ambion- life Technologies). DNMT3A knock-down was verified by RTPCR (TaqMan Applied Biosystem ABI7500; Taqman primer/probes for DNMT3A, Hs01027166_m1).
Results
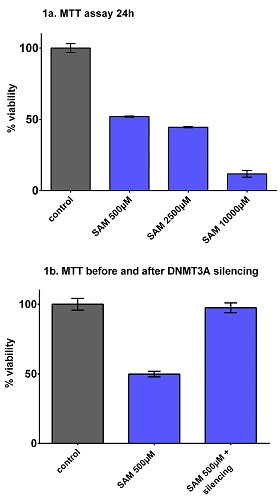
A significant dose dependent reduction of the cells viability was evident for SAM concentrations equal or superior to 500μM, with an IC50 of 500μM (figure 1a). Since a Cmax of 211μM (SD 94) after single intravenous infusion of SAM was previously reported in healthy volunteers, we tested SAM concentations close to 211μM, and observed a significant reduction of the cells viability with SAM 200μM (62,74% viable cells) and SAM 300μM (53.32% viable cells), (data not shown). The apoptosis assay at 24h and 72h of SAM treatment, showed no significant increase in the amount of apoptotic cells, (data not shown), however a dose dependent decrease of the percentages of living cells was observed which was considered congruent with the results of the MTT tests. The MTT assay was repeated testing at the same time DNMT3A silenced and non-silenced OCI-AML3 cells. SiRNA transfection efficiency was 80%. Gene knock-down was confirmed by a DNMT3A expression of 39% compared to the non-silenced cells. In the MTT assay with SAM 500 μM (24h) the non-silenced cells showed a significant reduction of the cell viability (49,8% viable cells), while no effect was observed in the DNMT3A silenced cells (97,5% viable cells) (figure 1b).
Summary
SAM shows in vitro cytostatic activity on OCI-AML3 cells, at concentrations close to the blood concentrations achievable in humans. These preliminary results are in line with other studies using similar SAM concentrations in solid tumors cell lines. The MTT assay performed after DNMT3A knock-down demonstrated that the cytostatic effect of SAM on OCI-AML3 cells is specifically mediated by DNMT3A. This finding suggests that modulating the activity of DNMT3A with supraphysiological concentrations of its substrate can induce cell growth inhibition in AML cells with heterozygous DNMT3A mutation. SAM should be further investigated as a potential antileukemic compound in AML with NPM1 and DNMT3A mutations.
Keyword(s): Acute myeloid leukemia, Epigenetic, Methylation, Therapy
Session topic: Publication Only
Type: Publication Only
Background
AML with mutated NPM1 and its cell line OCI-AML3 carry the NPM1 mutation A and the heterozygous R882C mutation of the DNA (cytosine-5-)-4 methyltransferase 3 alpha (DNMT3A). The mutant DNMT3A protein exerts a dominant negative inhibition on the wild type protein resulting in focal hypomethylation. Of note, AML with mutated NPM1 harbors a distinct methylation profile among AML subtypes. S-adenosylmethionine (SAM) is a universal methyl donor and a coenzyme of DNMT3A. SAM has been used in the clinical practice as an antidepressant drug with very limited side effects. There are growing evidences of the antineoplastic activity of SAM in vitro and in murine models of solid cancers. Recently, the combination of SAM with demethylating agents showed an interesting in vitro synergistic cytotoxicity on breast cancer cells.
Aims
To explore the effect of SAM on OCI-AML3 cells and its dependence on DNMT3A activity.
Methods
We used the MTT assay to test the cytostatic effect of SAM iodide (Sigma-Aldrich, St. Louis -MO) on OCI-AML3 cells (DSMZ Leibniz Institut, Braunschweig -Germany). The cells were treated with SAM for 24h. Untreated and treated cells were tested in triplicate for each SAM concentration. Results were expressed as percentage of cells viability compared to the control (i.e. OCI-AML3 untreated cells). The Apoptosis assays were performed after 24h and 72h of SAM treatment using the Tali® Apoptosis Assay Kit–Annexin V Alexa Fluor®488/Propidium Iodide (Invitrogen, Carlsbad, CA) for cells staining, and the Tali Image Based Cytometer (Invitrogen) for cells counting. In order to verify if the observed effect was mediated by DNMT3A, we repeated the MTT assay after DNMT3A silencing with a predesigned SiRNA directed against DNMT3A (SiRNA ID HSS176224, life technologies). Transfection was performed on triplicates cultures with lipofectamine RNAiMax (Invitrogen) and transfection efficiency calculated by BLOCK-IT AlexaFluor Red Fluorescent Oligo (Ambion- life Technologies). DNMT3A knock-down was verified by RTPCR (TaqMan Applied Biosystem ABI7500; Taqman primer/probes for DNMT3A, Hs01027166_m1).
Results
A significant dose dependent reduction of the cells viability was evident for SAM concentrations equal or superior to 500μM, with an IC50 of 500μM (figure 1a). Since a Cmax of 211μM (SD 94) after single intravenous infusion of SAM was previously reported in healthy volunteers, we tested SAM concentations close to 211μM, and observed a significant reduction of the cells viability with SAM 200μM (62,74% viable cells) and SAM 300μM (53.32% viable cells), (data not shown). The apoptosis assay at 24h and 72h of SAM treatment, showed no significant increase in the amount of apoptotic cells, (data not shown), however a dose dependent decrease of the percentages of living cells was observed which was considered congruent with the results of the MTT tests. The MTT assay was repeated testing at the same time DNMT3A silenced and non-silenced OCI-AML3 cells. SiRNA transfection efficiency was 80%. Gene knock-down was confirmed by a DNMT3A expression of 39% compared to the non-silenced cells. In the MTT assay with SAM 500 μM (24h) the non-silenced cells showed a significant reduction of the cell viability (49,8% viable cells), while no effect was observed in the DNMT3A silenced cells (97,5% viable cells) (figure 1b).
Summary
SAM shows in vitro cytostatic activity on OCI-AML3 cells, at concentrations close to the blood concentrations achievable in humans. These preliminary results are in line with other studies using similar SAM concentrations in solid tumors cell lines. The MTT assay performed after DNMT3A knock-down demonstrated that the cytostatic effect of SAM on OCI-AML3 cells is specifically mediated by DNMT3A. This finding suggests that modulating the activity of DNMT3A with supraphysiological concentrations of its substrate can induce cell growth inhibition in AML cells with heterozygous DNMT3A mutation. SAM should be further investigated as a potential antileukemic compound in AML with NPM1 and DNMT3A mutations.
Keyword(s): Acute myeloid leukemia, Epigenetic, Methylation, Therapy

Session topic: Publication Only


