National Health Service Spain

Contributions
Type: Publication Only
Background
Infectious complications occur in most patients receiving high dose therapy (HDT) and autologous stem cell transplantation (ASCT) both during neutropenia,related to the conditioning regimen and later on due to gradual immunological reconstitution of lymphoid and immune effector cells.
Fatal infections are rare, but they represent a mayor cause of morbidity and treatment related mortality.
Aims
We retrospectively analyzed the incidence and type of infections(early:day(d) 0-100,late:from d 100) after ASCT for haematological malignancies at our hospital from January 2002 to February 2015.
Methods
We studied 103 ASCT performed to 98 patients: 62 males and 41 females;median age:55 years (23-69); the diagnosis were: 42.7% Multiple Myeloma, Non Hodgkin Lymphoma(follicular 10.67%,Large cell B diffuse 10.67%,T 5.8%,anaplastic 0.97%,plasmablastic 0.97%),Hodgkin Lymphoma 19.4%,amyloidosis 1.9%, acute myeloid leukaemia 5.8%, acute lymphoblastic leukaemia 0.97%. Conditioning regimens were:BEAM 19.4%, escalated CBV 28.1%,melfalan 41.7%,BUMEL 2.9%,BEA 5.8%,other 1.9%.
All patients received antibiotic and antiviral prophylaxis during conditioning and for at least 6 months.Vaccinations were administered as stated in EBMT guidelines.(one dose of polysaccharide pneumococcal vaccine at 7m).
Results
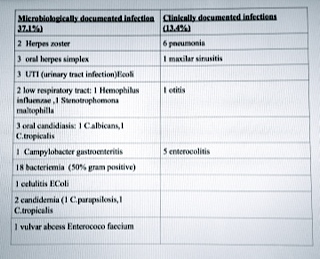
Early infections: 94.2% patients have febrile neutropenia; fever of unknown origin represented 44.3% of episodes, the documented infections were as follows:see table 1. We have a fatal sepsis caused by multirresistant pseudomonas aeruginosa .Mortality due to infection:1.03%.
Late infections: 75 episodes were recorded in 43 patients. Mortality due to infection was 7.14%.
The incidence of Herpes zoster reactivation was11.2% at 6m(3-48) postransplantation; 54.5% were receiving corticosteroids and 36.2% had relapsed. Urinary tract infections 12.2% at 57.5m(10-117)
Bacterial Pneumonia:20.4% at 43m(4-78);50% received corticosteroids and 65% had relapsed. Influenzae pneumonia 2%at 27.5 m(14-41).Invasive fungal infections(aspergillus) pneumonia 3.1%: 2 probable,1 proven at 31.5m(4-59) .Oropharyngeal candidiasis 3.1% at 45.5m (10-86).
Pneumococal infection: 8.2% at (45.5m (15-76):(5 invasive pneumococcal disease:1 arthritis, 3 bacteriemic pneumonia, 1meningitis), 3 pneumonia; 2 patients have multiple episodes;mortality rate was 25%;50%of patients had progressed.and 62.5% received corticosteroids.
Catheter related infections: 5.1% at 17m(4-46). Gastroenteritis 3.1%(1 campylobacter, 2 clostridium difficile) at 13m (7-23). 1(1.02%)episode of bacteriaemia by Listeria monocytogenes without focal infection at 8m. 1(1.02%)Visceral Leishmaniasis at 33m. Cryptosporidium infection 3.1% (1 systemic, 2 diarrhea) at 40.5m(8-73).
Summary
Our incidence of infectious complications during neutropenia period is similar to that previously described in literature. The incidence of fungal infections is low. 43.8% of our patients suffered late infections and the mortality was 16.2%.Herpes zoster is an important cause of morbidity late after ASCT.
Pneumococcal infections stand out due to its continuous occurrence late after transplantation and its high mortality; perhaps the use of the 13-valent pneumococcal conjugated vaccine could help to prevent it .
Constant surveillance and prompt therapy must be held for infections, even late after autologous transplantation.
Keyword(s): Autologous hematopoietic stem cell transplantation, Infection
Type: Publication Only
Background
Infectious complications occur in most patients receiving high dose therapy (HDT) and autologous stem cell transplantation (ASCT) both during neutropenia,related to the conditioning regimen and later on due to gradual immunological reconstitution of lymphoid and immune effector cells.
Fatal infections are rare, but they represent a mayor cause of morbidity and treatment related mortality.
Aims
We retrospectively analyzed the incidence and type of infections(early:day(d) 0-100,late:from d 100) after ASCT for haematological malignancies at our hospital from January 2002 to February 2015.
Methods
We studied 103 ASCT performed to 98 patients: 62 males and 41 females;median age:55 years (23-69); the diagnosis were: 42.7% Multiple Myeloma, Non Hodgkin Lymphoma(follicular 10.67%,Large cell B diffuse 10.67%,T 5.8%,anaplastic 0.97%,plasmablastic 0.97%),Hodgkin Lymphoma 19.4%,amyloidosis 1.9%, acute myeloid leukaemia 5.8%, acute lymphoblastic leukaemia 0.97%. Conditioning regimens were:BEAM 19.4%, escalated CBV 28.1%,melfalan 41.7%,BUMEL 2.9%,BEA 5.8%,other 1.9%.
All patients received antibiotic and antiviral prophylaxis during conditioning and for at least 6 months.Vaccinations were administered as stated in EBMT guidelines.(one dose of polysaccharide pneumococcal vaccine at 7m).
Results
Early infections: 94.2% patients have febrile neutropenia; fever of unknown origin represented 44.3% of episodes, the documented infections were as follows:see table 1. We have a fatal sepsis caused by multirresistant pseudomonas aeruginosa .Mortality due to infection:1.03%.
Late infections: 75 episodes were recorded in 43 patients. Mortality due to infection was 7.14%.
The incidence of Herpes zoster reactivation was11.2% at 6m(3-48) postransplantation; 54.5% were receiving corticosteroids and 36.2% had relapsed. Urinary tract infections 12.2% at 57.5m(10-117)
Bacterial Pneumonia:20.4% at 43m(4-78);50% received corticosteroids and 65% had relapsed. Influenzae pneumonia 2%at 27.5 m(14-41).Invasive fungal infections(aspergillus) pneumonia 3.1%: 2 probable,1 proven at 31.5m(4-59) .Oropharyngeal candidiasis 3.1% at 45.5m (10-86).
Pneumococal infection: 8.2% at (45.5m (15-76):(5 invasive pneumococcal disease:1 arthritis, 3 bacteriemic pneumonia, 1meningitis), 3 pneumonia; 2 patients have multiple episodes;mortality rate was 25%;50%of patients had progressed.and 62.5% received corticosteroids.
Catheter related infections: 5.1% at 17m(4-46). Gastroenteritis 3.1%(1 campylobacter, 2 clostridium difficile) at 13m (7-23). 1(1.02%)episode of bacteriaemia by Listeria monocytogenes without focal infection at 8m. 1(1.02%)Visceral Leishmaniasis at 33m. Cryptosporidium infection 3.1% (1 systemic, 2 diarrhea) at 40.5m(8-73).
Summary
Our incidence of infectious complications during neutropenia period is similar to that previously described in literature. The incidence of fungal infections is low. 43.8% of our patients suffered late infections and the mortality was 16.2%.Herpes zoster is an important cause of morbidity late after ASCT.
Pneumococcal infections stand out due to its continuous occurrence late after transplantation and its high mortality; perhaps the use of the 13-valent pneumococcal conjugated vaccine could help to prevent it .
Constant surveillance and prompt therapy must be held for infections, even late after autologous transplantation.
Keyword(s): Autologous hematopoietic stem cell transplantation, Infection



