
Contributions
Type: Publication Only
Background
In thalassemia intermedia (TI) patients no observational study prospectively evaluated in the real life the efficacy of the desferrioxamine (DFO) therapy in removing or preventing iron overload from the heart and the liver by T2* Magnetic Resonance Imaging (MRI).
Aims
The efficacy endpoint of this study is represented by the changes in cardiac T2* values, MRI LIC (liver iron concentration) values and biventricular function parameters in non-transfusion dependent (NTD) TI patients after 18 months of desferrioxamine therapy.
Methods
Among the 325 TI patients enrolled in the MIOT (Myocardial Iron Overload in Thalassemia) network, we selected 129 TI patients NTD. We considered 29 patients who had been received DFO alone between the two MRI scans. Cardiac iron overload was assessed by the multislice multiecho T2* technique. Hepatic T2* values were assessed in a homogeneous tissue area and converted into LIC. Biventricular function parameters were quantified by cine SSFP sequences. Myocardial fibrosis was evaluated by late gadolinium enhancement (LGE) acquisitions.
Results
Mean age was 39.69±8.12 years and 14 (48.3%) patients were females. Patients started regular chelation therapy at a mean age of 21.92±15.89 years. The mean administered dosage of DFO via subcutaneous route was 38.46±10.27 mg/kg body weight on 3.32±1.54 days/week. The percentage of patients with excellent/good levels of compliance to the chelation treatment was 82.1%.
At baseline only one patient showed cardiac iron overload (global heart T2*=15.23 ms) but he recovered at the FU (global heart T2*=26.93 ms). All patients without cardiac iron maintained the same status at the follow-up (FU).
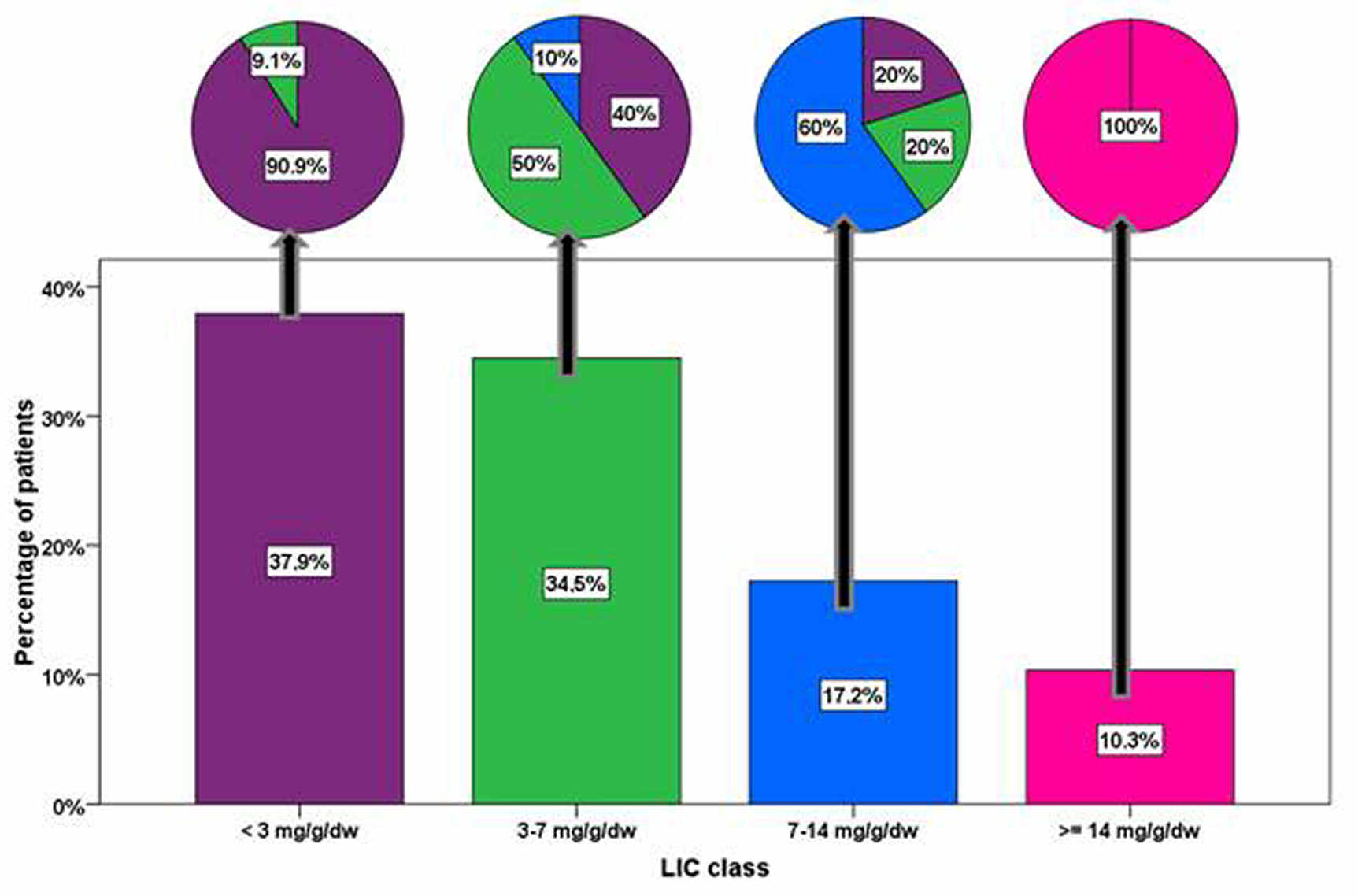
Eighteen patients (62.1%) had hepatic iron overload (MRI LIC ≥3 mg/g/dw) at the baseline. For this subgroup, the baseline and the FU LIC values were, respectively, 9.15 ± 7.97 mg/g/dw and 7.41 ± 6.28 mg/g/dw. The reduction in MRI LIC values was not significant (P=0.102). Out of the 11 patients with a baseline MRI LIC <3 mg/g/dw, only one (9.1%) showed hepatic iron at the FU. The Figure shows the evolution of different hepatic iron overload risk classes between the baseline and the FU.
Due mainly to technical reasons, cardiac function was assessed at both baseline and FU MRIs in 24 patients. At baseline all patients had a normal LV ejection fraction (EF) and 4 of them showed a reduced LV ejection fraction (LVEF) at the FU. No patient had a pathological RV EF. No significant change between the two MRIs was detected in biventricular volume indexes, biventricular EFs and LV mass index.
For 21 patients the presence of myocardial fibrosis was investigated at both baseline and FU MRIs, and this subgroup was considered. Three (14.3%) patients had myocardial fibrosis at the baseline, all with a non ischemic pattern. At the FU two new occurrences of non-ischemic myocardial fibrosis were detected.
Summary
In this small population of sporadically or non transfused TI patients, the DFO therapy showed 100% efficacy in maintaining a normal global heart T2* value. As regards as the hepatic iron overload, the DFO therapy did not prevent the transition to a worst class in 2 patients. Moreover, the DFO therapy did not prevent the worsening of the LV function and the occurrence of new myocardial fibrosis.
Keyword(s): Chelation, Iron overload, Magnetic resonance imaging, Thalassemia
Type: Publication Only
Background
In thalassemia intermedia (TI) patients no observational study prospectively evaluated in the real life the efficacy of the desferrioxamine (DFO) therapy in removing or preventing iron overload from the heart and the liver by T2* Magnetic Resonance Imaging (MRI).
Aims
The efficacy endpoint of this study is represented by the changes in cardiac T2* values, MRI LIC (liver iron concentration) values and biventricular function parameters in non-transfusion dependent (NTD) TI patients after 18 months of desferrioxamine therapy.
Methods
Among the 325 TI patients enrolled in the MIOT (Myocardial Iron Overload in Thalassemia) network, we selected 129 TI patients NTD. We considered 29 patients who had been received DFO alone between the two MRI scans. Cardiac iron overload was assessed by the multislice multiecho T2* technique. Hepatic T2* values were assessed in a homogeneous tissue area and converted into LIC. Biventricular function parameters were quantified by cine SSFP sequences. Myocardial fibrosis was evaluated by late gadolinium enhancement (LGE) acquisitions.
Results
Mean age was 39.69±8.12 years and 14 (48.3%) patients were females. Patients started regular chelation therapy at a mean age of 21.92±15.89 years. The mean administered dosage of DFO via subcutaneous route was 38.46±10.27 mg/kg body weight on 3.32±1.54 days/week. The percentage of patients with excellent/good levels of compliance to the chelation treatment was 82.1%.
At baseline only one patient showed cardiac iron overload (global heart T2*=15.23 ms) but he recovered at the FU (global heart T2*=26.93 ms). All patients without cardiac iron maintained the same status at the follow-up (FU).
Eighteen patients (62.1%) had hepatic iron overload (MRI LIC ≥3 mg/g/dw) at the baseline. For this subgroup, the baseline and the FU LIC values were, respectively, 9.15 ± 7.97 mg/g/dw and 7.41 ± 6.28 mg/g/dw. The reduction in MRI LIC values was not significant (P=0.102). Out of the 11 patients with a baseline MRI LIC <3 mg/g/dw, only one (9.1%) showed hepatic iron at the FU. The Figure shows the evolution of different hepatic iron overload risk classes between the baseline and the FU.
Due mainly to technical reasons, cardiac function was assessed at both baseline and FU MRIs in 24 patients. At baseline all patients had a normal LV ejection fraction (EF) and 4 of them showed a reduced LV ejection fraction (LVEF) at the FU. No patient had a pathological RV EF. No significant change between the two MRIs was detected in biventricular volume indexes, biventricular EFs and LV mass index.
For 21 patients the presence of myocardial fibrosis was investigated at both baseline and FU MRIs, and this subgroup was considered. Three (14.3%) patients had myocardial fibrosis at the baseline, all with a non ischemic pattern. At the FU two new occurrences of non-ischemic myocardial fibrosis were detected.
Summary
In this small population of sporadically or non transfused TI patients, the DFO therapy showed 100% efficacy in maintaining a normal global heart T2* value. As regards as the hepatic iron overload, the DFO therapy did not prevent the transition to a worst class in 2 patients. Moreover, the DFO therapy did not prevent the worsening of the LV function and the occurrence of new myocardial fibrosis.
Keyword(s): Chelation, Iron overload, Magnetic resonance imaging, Thalassemia



