OPROZOMIB (OPZ) AND DEXAMETHASONE (DEX) IN PATIENTS (PTS) WITH RELAPSED AND/OR REFRACTORY MULTIPLE MYELOMA (RRMM): UPDATED RESULTS FROM DOSE ESCALATION IN A PHASE 1B/2, MULTICENTER, OPEN-LABEL STUDY
(Abstract release date: 05/21/15)
EHA Library. HARI P. 06/13/15; 100794; P653
Disclosure(s): Medical College of Wisconsin
Prof. PARAMESWARAN HARI
Contributions
Contributions
Abstract
Abstract: P653
Type: Poster Presentation
Presentation during EHA20: From 13.06.2015 17:15 to 13.06.2015 18:45
Location: Poster area (Hall C)
Background
The oral proteasome inhibitor OPZ has shown promising antitumor activity in pts with hematologic malignancies, including multiple myeloma (MM; Vij, Blood 2014;121:abstract 34).
Aims
We present updated results from an ongoing, single-arm, phase 1b/2 study (NCT01832727) that is evaluating the safety and tolerability of OPZ with DEX in pts with RRMM.
Methods
Pts with RRMM who have received 1–5 prior lines of therapy (≥1 regimen including lenalidomide and/or bortezomib) are eligible. Pts are receiving OPZ tablets (PO) on days 1, 2, 8, and 9 (2/7 schedule) or on days 1–5 (5/14 schedule) of a 14-day cycle. All pts are receiving DEX (20 mg PO) on days 1, 2, 8, and 9. Treatment is being administered until pt withdrawal, disease progression, or unacceptable toxicity. The starting OPZ dose was 210 mg on both schedules. OPZ doses are being escalated in 30-mg increments using standard 3+3 dose escalation. The primary objectives of the phase 1b study are to determine the maximum tolerated dose (MTD), recommend the phase 2 dose (RP2D) of OPZ with DEX, and to evaluate safety and tolerability. Response is being assessed by IMWG criteria, with minimal response (MR) and near complete response defined by modified EBMT criteria. Safety is being assessed by CTCAE, v4.03. All pts provided informed consent.
Results
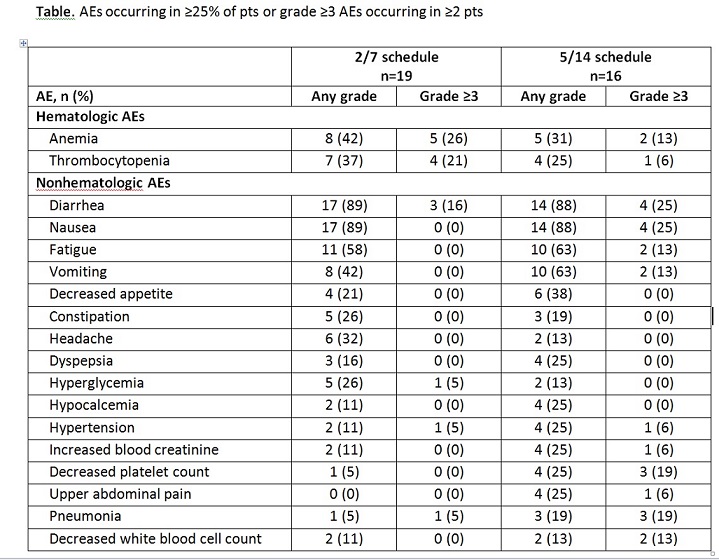
As of January 19, 2015, 22 and 19 pts were enrolled on the 2/7 and 5/14 schedules, respectively. Preliminary data are available for a total of 35 pts enrolled as of November 3, 2014 (2/7 schedule, n=19; 5/14 schedule, n=16), 31 of whom were evaluable for response (2/7 schedule, n=17; 5/14 schedule, n=14). The median age of pts was 63 years (2/7 schedule) and 63.5 years (5/14 schedule). Pts received a median of 3 (range, 1–5) prior regimens in the 2/7 schedule and 2 (range, 1–5) prior regimens in the 5/14 schedule. Preliminary median OPZ treatment duration was 15 weeks in the 2/7 schedule (range, 1.3–51.3 weeks) and 5.7 weeks in the 5/14 schedule (range, 0.7–24.7 weeks). No dose-limiting toxicities (DLTs) occurred in the 2/7 schedule. In the 5/14 schedule, 3 DLTs occurred (210-mg/day dosing level: grade 2 subarachnoid hemorrhage; grade 3 transaminitis; and grade 4 thrombocytopenia); the MTD was 180 mg/day. The MTD was not reached in the 2/7 schedule up to a dosing level of 300 mg/day. In both schedules combined, the most common adverse events (AEs) of any grade were diarrhea, nausea, and fatigue; the most common grade ≥3 AEs were anemia, diarrhea, and thrombocytopenia (Table). Two grade 5 AEs of sepsis occurred: 1 in the 2/7 schedule (240 mg/day) and 1 in the 5/14 schedule (210 mg/day). Three pts on the 2/7 schedule and 9 pts on the 5/14 schedule discontinued treatment due to an AE. Five pts in each schedule had their OPZ dose reduced due to an AE. On the 2/7 schedule, the overall response rate (ORR; ≥partial response [PR]) was 35.3% and the clinical benefit rate (CBR; ≥MR) was 41.2% (6 pts had a PR, 1 had an MR, and 8 had stable disease [SD]). On the 5/14 schedule, the ORR was 7.1% and the CBR was 35.7% (1 pt had a PR, 4 had an MR, and 6 had SD).
Summary
In the 5/14 schedule, the MTD was 180 mg/day; the MTD was not reached in the 2/7 schedule. Preliminary results suggest that OPZ with DEX has promising antitumor activity in pts with RRMM receiving the 2/7 schedule; this schedule has been selected as the recommended phase 2 schedule. Dose escalation will continue in the 2/7 schedule until the MTD or RP2D is determined. Updated data (including pharmacokinetics) for both schedules will be presented at the meeting.
Keyword(s): Multiple myeloma, Phase I/II, Proteasome inhibitor
Session topic: Multiple myeloma - Clinical 3
Type: Poster Presentation
Presentation during EHA20: From 13.06.2015 17:15 to 13.06.2015 18:45
Location: Poster area (Hall C)
Background
The oral proteasome inhibitor OPZ has shown promising antitumor activity in pts with hematologic malignancies, including multiple myeloma (MM; Vij, Blood 2014;121:abstract 34).
Aims
We present updated results from an ongoing, single-arm, phase 1b/2 study (NCT01832727) that is evaluating the safety and tolerability of OPZ with DEX in pts with RRMM.
Methods
Pts with RRMM who have received 1–5 prior lines of therapy (≥1 regimen including lenalidomide and/or bortezomib) are eligible. Pts are receiving OPZ tablets (PO) on days 1, 2, 8, and 9 (2/7 schedule) or on days 1–5 (5/14 schedule) of a 14-day cycle. All pts are receiving DEX (20 mg PO) on days 1, 2, 8, and 9. Treatment is being administered until pt withdrawal, disease progression, or unacceptable toxicity. The starting OPZ dose was 210 mg on both schedules. OPZ doses are being escalated in 30-mg increments using standard 3+3 dose escalation. The primary objectives of the phase 1b study are to determine the maximum tolerated dose (MTD), recommend the phase 2 dose (RP2D) of OPZ with DEX, and to evaluate safety and tolerability. Response is being assessed by IMWG criteria, with minimal response (MR) and near complete response defined by modified EBMT criteria. Safety is being assessed by CTCAE, v4.03. All pts provided informed consent.
Results
As of January 19, 2015, 22 and 19 pts were enrolled on the 2/7 and 5/14 schedules, respectively. Preliminary data are available for a total of 35 pts enrolled as of November 3, 2014 (2/7 schedule, n=19; 5/14 schedule, n=16), 31 of whom were evaluable for response (2/7 schedule, n=17; 5/14 schedule, n=14). The median age of pts was 63 years (2/7 schedule) and 63.5 years (5/14 schedule). Pts received a median of 3 (range, 1–5) prior regimens in the 2/7 schedule and 2 (range, 1–5) prior regimens in the 5/14 schedule. Preliminary median OPZ treatment duration was 15 weeks in the 2/7 schedule (range, 1.3–51.3 weeks) and 5.7 weeks in the 5/14 schedule (range, 0.7–24.7 weeks). No dose-limiting toxicities (DLTs) occurred in the 2/7 schedule. In the 5/14 schedule, 3 DLTs occurred (210-mg/day dosing level: grade 2 subarachnoid hemorrhage; grade 3 transaminitis; and grade 4 thrombocytopenia); the MTD was 180 mg/day. The MTD was not reached in the 2/7 schedule up to a dosing level of 300 mg/day. In both schedules combined, the most common adverse events (AEs) of any grade were diarrhea, nausea, and fatigue; the most common grade ≥3 AEs were anemia, diarrhea, and thrombocytopenia (Table). Two grade 5 AEs of sepsis occurred: 1 in the 2/7 schedule (240 mg/day) and 1 in the 5/14 schedule (210 mg/day). Three pts on the 2/7 schedule and 9 pts on the 5/14 schedule discontinued treatment due to an AE. Five pts in each schedule had their OPZ dose reduced due to an AE. On the 2/7 schedule, the overall response rate (ORR; ≥partial response [PR]) was 35.3% and the clinical benefit rate (CBR; ≥MR) was 41.2% (6 pts had a PR, 1 had an MR, and 8 had stable disease [SD]). On the 5/14 schedule, the ORR was 7.1% and the CBR was 35.7% (1 pt had a PR, 4 had an MR, and 6 had SD).
Summary
In the 5/14 schedule, the MTD was 180 mg/day; the MTD was not reached in the 2/7 schedule. Preliminary results suggest that OPZ with DEX has promising antitumor activity in pts with RRMM receiving the 2/7 schedule; this schedule has been selected as the recommended phase 2 schedule. Dose escalation will continue in the 2/7 schedule until the MTD or RP2D is determined. Updated data (including pharmacokinetics) for both schedules will be presented at the meeting.
Keyword(s): Multiple myeloma, Phase I/II, Proteasome inhibitor

Session topic: Multiple myeloma - Clinical 3
Abstract: P653
Type: Poster Presentation
Presentation during EHA20: From 13.06.2015 17:15 to 13.06.2015 18:45
Location: Poster area (Hall C)
Background
The oral proteasome inhibitor OPZ has shown promising antitumor activity in pts with hematologic malignancies, including multiple myeloma (MM; Vij, Blood 2014;121:abstract 34).
Aims
We present updated results from an ongoing, single-arm, phase 1b/2 study (NCT01832727) that is evaluating the safety and tolerability of OPZ with DEX in pts with RRMM.
Methods
Pts with RRMM who have received 1–5 prior lines of therapy (≥1 regimen including lenalidomide and/or bortezomib) are eligible. Pts are receiving OPZ tablets (PO) on days 1, 2, 8, and 9 (2/7 schedule) or on days 1–5 (5/14 schedule) of a 14-day cycle. All pts are receiving DEX (20 mg PO) on days 1, 2, 8, and 9. Treatment is being administered until pt withdrawal, disease progression, or unacceptable toxicity. The starting OPZ dose was 210 mg on both schedules. OPZ doses are being escalated in 30-mg increments using standard 3+3 dose escalation. The primary objectives of the phase 1b study are to determine the maximum tolerated dose (MTD), recommend the phase 2 dose (RP2D) of OPZ with DEX, and to evaluate safety and tolerability. Response is being assessed by IMWG criteria, with minimal response (MR) and near complete response defined by modified EBMT criteria. Safety is being assessed by CTCAE, v4.03. All pts provided informed consent.
Results
As of January 19, 2015, 22 and 19 pts were enrolled on the 2/7 and 5/14 schedules, respectively. Preliminary data are available for a total of 35 pts enrolled as of November 3, 2014 (2/7 schedule, n=19; 5/14 schedule, n=16), 31 of whom were evaluable for response (2/7 schedule, n=17; 5/14 schedule, n=14). The median age of pts was 63 years (2/7 schedule) and 63.5 years (5/14 schedule). Pts received a median of 3 (range, 1–5) prior regimens in the 2/7 schedule and 2 (range, 1–5) prior regimens in the 5/14 schedule. Preliminary median OPZ treatment duration was 15 weeks in the 2/7 schedule (range, 1.3–51.3 weeks) and 5.7 weeks in the 5/14 schedule (range, 0.7–24.7 weeks). No dose-limiting toxicities (DLTs) occurred in the 2/7 schedule. In the 5/14 schedule, 3 DLTs occurred (210-mg/day dosing level: grade 2 subarachnoid hemorrhage; grade 3 transaminitis; and grade 4 thrombocytopenia); the MTD was 180 mg/day. The MTD was not reached in the 2/7 schedule up to a dosing level of 300 mg/day. In both schedules combined, the most common adverse events (AEs) of any grade were diarrhea, nausea, and fatigue; the most common grade ≥3 AEs were anemia, diarrhea, and thrombocytopenia (Table). Two grade 5 AEs of sepsis occurred: 1 in the 2/7 schedule (240 mg/day) and 1 in the 5/14 schedule (210 mg/day). Three pts on the 2/7 schedule and 9 pts on the 5/14 schedule discontinued treatment due to an AE. Five pts in each schedule had their OPZ dose reduced due to an AE. On the 2/7 schedule, the overall response rate (ORR; ≥partial response [PR]) was 35.3% and the clinical benefit rate (CBR; ≥MR) was 41.2% (6 pts had a PR, 1 had an MR, and 8 had stable disease [SD]). On the 5/14 schedule, the ORR was 7.1% and the CBR was 35.7% (1 pt had a PR, 4 had an MR, and 6 had SD).
Summary
In the 5/14 schedule, the MTD was 180 mg/day; the MTD was not reached in the 2/7 schedule. Preliminary results suggest that OPZ with DEX has promising antitumor activity in pts with RRMM receiving the 2/7 schedule; this schedule has been selected as the recommended phase 2 schedule. Dose escalation will continue in the 2/7 schedule until the MTD or RP2D is determined. Updated data (including pharmacokinetics) for both schedules will be presented at the meeting.
Keyword(s): Multiple myeloma, Phase I/II, Proteasome inhibitor

Session topic: Multiple myeloma - Clinical 3
Type: Poster Presentation
Presentation during EHA20: From 13.06.2015 17:15 to 13.06.2015 18:45
Location: Poster area (Hall C)
Background
The oral proteasome inhibitor OPZ has shown promising antitumor activity in pts with hematologic malignancies, including multiple myeloma (MM; Vij, Blood 2014;121:abstract 34).
Aims
We present updated results from an ongoing, single-arm, phase 1b/2 study (NCT01832727) that is evaluating the safety and tolerability of OPZ with DEX in pts with RRMM.
Methods
Pts with RRMM who have received 1–5 prior lines of therapy (≥1 regimen including lenalidomide and/or bortezomib) are eligible. Pts are receiving OPZ tablets (PO) on days 1, 2, 8, and 9 (2/7 schedule) or on days 1–5 (5/14 schedule) of a 14-day cycle. All pts are receiving DEX (20 mg PO) on days 1, 2, 8, and 9. Treatment is being administered until pt withdrawal, disease progression, or unacceptable toxicity. The starting OPZ dose was 210 mg on both schedules. OPZ doses are being escalated in 30-mg increments using standard 3+3 dose escalation. The primary objectives of the phase 1b study are to determine the maximum tolerated dose (MTD), recommend the phase 2 dose (RP2D) of OPZ with DEX, and to evaluate safety and tolerability. Response is being assessed by IMWG criteria, with minimal response (MR) and near complete response defined by modified EBMT criteria. Safety is being assessed by CTCAE, v4.03. All pts provided informed consent.
Results
As of January 19, 2015, 22 and 19 pts were enrolled on the 2/7 and 5/14 schedules, respectively. Preliminary data are available for a total of 35 pts enrolled as of November 3, 2014 (2/7 schedule, n=19; 5/14 schedule, n=16), 31 of whom were evaluable for response (2/7 schedule, n=17; 5/14 schedule, n=14). The median age of pts was 63 years (2/7 schedule) and 63.5 years (5/14 schedule). Pts received a median of 3 (range, 1–5) prior regimens in the 2/7 schedule and 2 (range, 1–5) prior regimens in the 5/14 schedule. Preliminary median OPZ treatment duration was 15 weeks in the 2/7 schedule (range, 1.3–51.3 weeks) and 5.7 weeks in the 5/14 schedule (range, 0.7–24.7 weeks). No dose-limiting toxicities (DLTs) occurred in the 2/7 schedule. In the 5/14 schedule, 3 DLTs occurred (210-mg/day dosing level: grade 2 subarachnoid hemorrhage; grade 3 transaminitis; and grade 4 thrombocytopenia); the MTD was 180 mg/day. The MTD was not reached in the 2/7 schedule up to a dosing level of 300 mg/day. In both schedules combined, the most common adverse events (AEs) of any grade were diarrhea, nausea, and fatigue; the most common grade ≥3 AEs were anemia, diarrhea, and thrombocytopenia (Table). Two grade 5 AEs of sepsis occurred: 1 in the 2/7 schedule (240 mg/day) and 1 in the 5/14 schedule (210 mg/day). Three pts on the 2/7 schedule and 9 pts on the 5/14 schedule discontinued treatment due to an AE. Five pts in each schedule had their OPZ dose reduced due to an AE. On the 2/7 schedule, the overall response rate (ORR; ≥partial response [PR]) was 35.3% and the clinical benefit rate (CBR; ≥MR) was 41.2% (6 pts had a PR, 1 had an MR, and 8 had stable disease [SD]). On the 5/14 schedule, the ORR was 7.1% and the CBR was 35.7% (1 pt had a PR, 4 had an MR, and 6 had SD).
Summary
In the 5/14 schedule, the MTD was 180 mg/day; the MTD was not reached in the 2/7 schedule. Preliminary results suggest that OPZ with DEX has promising antitumor activity in pts with RRMM receiving the 2/7 schedule; this schedule has been selected as the recommended phase 2 schedule. Dose escalation will continue in the 2/7 schedule until the MTD or RP2D is determined. Updated data (including pharmacokinetics) for both schedules will be presented at the meeting.
Keyword(s): Multiple myeloma, Phase I/II, Proteasome inhibitor

Session topic: Multiple myeloma - Clinical 3
{{ help_message }}
{{filter}}


