
Contributions
Abstract: EP1211
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
VOCs are the most common cause of SCD-related hospitalization and can lead to serious complications. Hydroxyurea (HU) has historically been a mainstay for VOC prevention, but new therapies are now available. Crizanlizumab is an anti-P-selectin monoclonal antibody approved in several regions to prevent/reduce VOC burden for SCD patients aged ≥16 years. Early access to crizanlizumab is possible before health authority approval in some countries via a MAP (NCT03720626). Baseline patient data are collected to assess their eligibility for MAP participation. We conducted a descriptive analysis of aggregated baseline data for patients eligible to enter the MAP in countries where publication of these data was allowed.
Aims
We analyzed baseline SCD characteristics and treatment history for patients participating in the MAP to assess real-world disease burden prior to receipt of crizanlizumab. The data set primarily included patients in Brazil, but also included those in Canada, Israel, Italy and Spain.
Methods
The MAP was designed to provide access to crizanlizumab for patients with serious or life-threatening disease for which no comparable or satisfactory alternative to crizanlizumab was available as treatment in their country. Crizanlizumab access was sought via independent request by each patient’s physician. Other patient eligibility criteria were: age 16–70 years (18–70 years in Italy); confirmed SCD (any genotype); history of VOCs as assessed by the treating physician (including recurrent VOCs while receiving preventative therapies like HU); not eligible for a crizanlizumab clinical trial; and not participating in a chronic transfusion program (simple or exchange).
Results
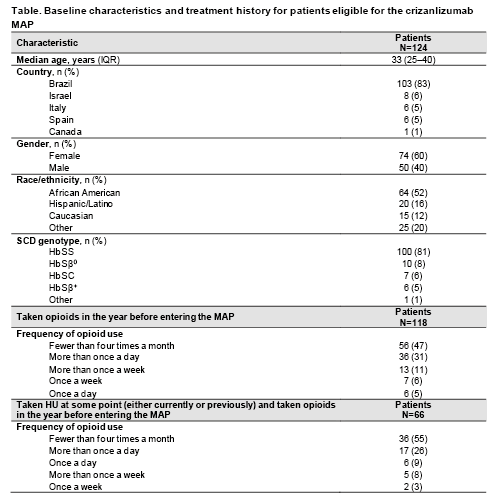
As of 31 January 2021, baseline data were available for 124 patients eligible for this analysis. Median age was 33 years, 60% were female and 83% were from Brazil (Table). In the year before entering the MAP, 122 patients (98%) had experienced ≥1 home-managed VOC (median 6, interquartile range [IQR] 4–10), 118 (95%) had experienced ≥1 healthcare-managed VOC (median 4, IQR 2–5) and 106 (85%) had been hospitalized for SCD-related reasons (median 3, IQR 2–4).
Opioids had been taken by 118 patients (95%) in the year before entering the MAP; all of them took opioids for VOC-related pain. Of the 118 patients who took opioids, 47% took them <4 times a month, but 31% took them more than once a day (Table). Some physicians chose to provide specific HU-use information, for 93 patients, to help better characterize those patients’ medical history. Of these patients, 24 (26%) had never taken HU and 69 (74%) had, either ongoing at baseline or at any prior point. Of those 69 patients, 66 (96%) reported opioid use in the year prior to MAP entry. Of those 66 patients, 55% took opioids <4 times a month, but 26% took opioids more than once a day (Table).
Conclusion
By analyzing baseline characteristics in this patient population prior to crizanlizumab treatment in the MAP, we observed a high burden of home- and healthcare-managed VOCs and SCD-related hospitalization. This high disease burden was seen despite many patients reporting use of HU and/or opioids, which illustrates their unmet medical need, hence their request to participate in the MAP. These real-world data demonstrate the need for alternative treatments targeting VOC burden for SCD patients, beyond HU. Reduction in VOC frequency would also be expected to reduce the need for opioids to manage acute pain. Additional data are required to assess if these observations are replicated in other SCD patient populations.
Keyword(s): Sickle cell disease, Vasoocclusive crisis
Abstract: EP1211
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
VOCs are the most common cause of SCD-related hospitalization and can lead to serious complications. Hydroxyurea (HU) has historically been a mainstay for VOC prevention, but new therapies are now available. Crizanlizumab is an anti-P-selectin monoclonal antibody approved in several regions to prevent/reduce VOC burden for SCD patients aged ≥16 years. Early access to crizanlizumab is possible before health authority approval in some countries via a MAP (NCT03720626). Baseline patient data are collected to assess their eligibility for MAP participation. We conducted a descriptive analysis of aggregated baseline data for patients eligible to enter the MAP in countries where publication of these data was allowed.
Aims
We analyzed baseline SCD characteristics and treatment history for patients participating in the MAP to assess real-world disease burden prior to receipt of crizanlizumab. The data set primarily included patients in Brazil, but also included those in Canada, Israel, Italy and Spain.
Methods
The MAP was designed to provide access to crizanlizumab for patients with serious or life-threatening disease for which no comparable or satisfactory alternative to crizanlizumab was available as treatment in their country. Crizanlizumab access was sought via independent request by each patient’s physician. Other patient eligibility criteria were: age 16–70 years (18–70 years in Italy); confirmed SCD (any genotype); history of VOCs as assessed by the treating physician (including recurrent VOCs while receiving preventative therapies like HU); not eligible for a crizanlizumab clinical trial; and not participating in a chronic transfusion program (simple or exchange).
Results
As of 31 January 2021, baseline data were available for 124 patients eligible for this analysis. Median age was 33 years, 60% were female and 83% were from Brazil (Table). In the year before entering the MAP, 122 patients (98%) had experienced ≥1 home-managed VOC (median 6, interquartile range [IQR] 4–10), 118 (95%) had experienced ≥1 healthcare-managed VOC (median 4, IQR 2–5) and 106 (85%) had been hospitalized for SCD-related reasons (median 3, IQR 2–4).
Opioids had been taken by 118 patients (95%) in the year before entering the MAP; all of them took opioids for VOC-related pain. Of the 118 patients who took opioids, 47% took them <4 times a month, but 31% took them more than once a day (Table). Some physicians chose to provide specific HU-use information, for 93 patients, to help better characterize those patients’ medical history. Of these patients, 24 (26%) had never taken HU and 69 (74%) had, either ongoing at baseline or at any prior point. Of those 69 patients, 66 (96%) reported opioid use in the year prior to MAP entry. Of those 66 patients, 55% took opioids <4 times a month, but 26% took opioids more than once a day (Table).

Conclusion
By analyzing baseline characteristics in this patient population prior to crizanlizumab treatment in the MAP, we observed a high burden of home- and healthcare-managed VOCs and SCD-related hospitalization. This high disease burden was seen despite many patients reporting use of HU and/or opioids, which illustrates their unmet medical need, hence their request to participate in the MAP. These real-world data demonstrate the need for alternative treatments targeting VOC burden for SCD patients, beyond HU. Reduction in VOC frequency would also be expected to reduce the need for opioids to manage acute pain. Additional data are required to assess if these observations are replicated in other SCD patient populations.
Keyword(s): Sickle cell disease, Vasoocclusive crisis


