
Contributions
Abstract: PB1780
Type: Publication Only
Session title: Stem cell transplantation - Clinical
Background
Graft-vs-host disease (GvHD) may significantly impair the success rate of allogeneic stem cell transplantation. In pediatric patients, chronic GvHD (cGvHD) can lead to considerable morbidity, impaired quality of life and transplant-related mortality (Inagaki 2015). Although systemic corticosteroids are the standard of care in initial stages of moderate to severe cGvHD, there is little controlled trial evidence to indicate the best initial or second-line treatment strategy. In recently reported data from the phase 3 REACH3 study, ruxolitinib improved overall response rate (ORR) versus best available therapy in adults and adolescents (≥12 years of age) with steroid-refractory (SR) or steroid-dependent cGvHD (Zeiser ASH 2020 [abstract 77]; NCT03112603). Furthermore, ruxolitinib has demonstrated encouraging activity in SR cGvHD pediatric patients in case series and a single center study (González Vicent 2019, Moiseev 2020). These data, combined with knowledge of the role played by janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling in cGvHD pathophysiology, support the investigation of ruxolitinib in the pediatric cGvHD patients.
Aims
The REACH5 study will assess the safety, efficacy, and pharmacokinetics (PK) of ruxolitinib in pediatric patients with moderate to severe treatment-naive or SR cGvHD.
Methods
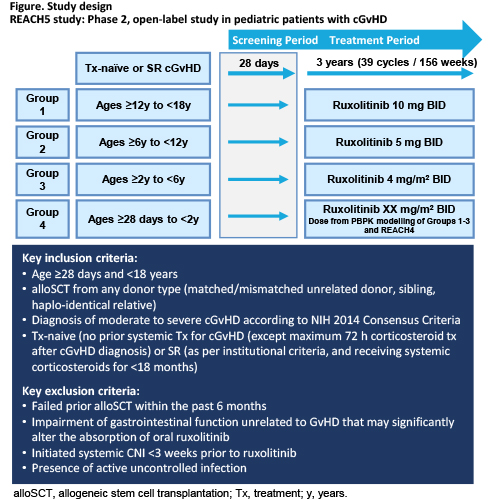
REACH5 is a Phase 2 open-label, single-arm, multicenter study of pediatric patients aged ≥28 days to <18 years divided into four age groups (see Figure for details). Patients are eligible for inclusion if diagnosed with moderate to severe cGvHD (National Institutes of Health [NIH] 2014 Consensus Criteria) and are either treatment-naïve or SR (as per institutional criteria and still receiving systemic corticosteroids). Patients will be treated for up to approximately 3 years (39 cycles/Week 156). The primary objective is to evaluate the activity of ruxolitinib twice daily (BID) added to corticosteroids +/- calcineurin inhibitor (CNI) measured by ORR at Week 24. Secondary endpoints include ruxolitinib safety, PK in treatment-naive and SR cGVHD, percentage of participants with ≥50% reduction from baseline in daily corticosteroid dose at Cycle 7 Day 1, duration of response, best overall response and failure-free survival. The study will enroll a minimum of 5 evaluable patients in Groups 1, 2 and 3, no minimum number of evaluable subjects in Group 4, and no cap to enrollment in any group. Group 1 patients will be treated at 10 mg BID, which is already considered the recommended phase 2 dose for this age group. Based on a review of PK data in the same aged group of patients treated in the pediatric acute GvHD study REACH4 [NCT03491215], the Group 2 dose has been confirmed at 5 mg BID and Group 3 dose at 4 mg/m2 BID. Enrollment initiation into Group 4 will be subject to the availability of data in this age group from REACH4, as well as a review of available PK, safety, and activity data generated from Groups 1 to 3 in the current study.
Results
Trial in progress.
Conclusion
As of February 2021, 25 patients have been enrolled. Enrollment is ongoing for Groups 1 and 2, with Group 3 opening in 2021 (ClinicalTrials.gov: NCT03774082).
Keyword(s): Chronic graft-versus-host, Graft-versus-host disease (GVHD), Pediatric, Ruxolitinib
Abstract: PB1780
Type: Publication Only
Session title: Stem cell transplantation - Clinical
Background
Graft-vs-host disease (GvHD) may significantly impair the success rate of allogeneic stem cell transplantation. In pediatric patients, chronic GvHD (cGvHD) can lead to considerable morbidity, impaired quality of life and transplant-related mortality (Inagaki 2015). Although systemic corticosteroids are the standard of care in initial stages of moderate to severe cGvHD, there is little controlled trial evidence to indicate the best initial or second-line treatment strategy. In recently reported data from the phase 3 REACH3 study, ruxolitinib improved overall response rate (ORR) versus best available therapy in adults and adolescents (≥12 years of age) with steroid-refractory (SR) or steroid-dependent cGvHD (Zeiser ASH 2020 [abstract 77]; NCT03112603). Furthermore, ruxolitinib has demonstrated encouraging activity in SR cGvHD pediatric patients in case series and a single center study (González Vicent 2019, Moiseev 2020). These data, combined with knowledge of the role played by janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling in cGvHD pathophysiology, support the investigation of ruxolitinib in the pediatric cGvHD patients.
Aims
The REACH5 study will assess the safety, efficacy, and pharmacokinetics (PK) of ruxolitinib in pediatric patients with moderate to severe treatment-naive or SR cGvHD.
Methods
REACH5 is a Phase 2 open-label, single-arm, multicenter study of pediatric patients aged ≥28 days to <18 years divided into four age groups (see Figure for details). Patients are eligible for inclusion if diagnosed with moderate to severe cGvHD (National Institutes of Health [NIH] 2014 Consensus Criteria) and are either treatment-naïve or SR (as per institutional criteria and still receiving systemic corticosteroids). Patients will be treated for up to approximately 3 years (39 cycles/Week 156). The primary objective is to evaluate the activity of ruxolitinib twice daily (BID) added to corticosteroids +/- calcineurin inhibitor (CNI) measured by ORR at Week 24. Secondary endpoints include ruxolitinib safety, PK in treatment-naive and SR cGVHD, percentage of participants with ≥50% reduction from baseline in daily corticosteroid dose at Cycle 7 Day 1, duration of response, best overall response and failure-free survival. The study will enroll a minimum of 5 evaluable patients in Groups 1, 2 and 3, no minimum number of evaluable subjects in Group 4, and no cap to enrollment in any group. Group 1 patients will be treated at 10 mg BID, which is already considered the recommended phase 2 dose for this age group. Based on a review of PK data in the same aged group of patients treated in the pediatric acute GvHD study REACH4 [NCT03491215], the Group 2 dose has been confirmed at 5 mg BID and Group 3 dose at 4 mg/m2 BID. Enrollment initiation into Group 4 will be subject to the availability of data in this age group from REACH4, as well as a review of available PK, safety, and activity data generated from Groups 1 to 3 in the current study.
Results
Trial in progress.

Conclusion
As of February 2021, 25 patients have been enrolled. Enrollment is ongoing for Groups 1 and 2, with Group 3 opening in 2021 (ClinicalTrials.gov: NCT03774082).
Keyword(s): Chronic graft-versus-host, Graft-versus-host disease (GVHD), Pediatric, Ruxolitinib


