
Contributions
Abstract: PB1713
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Anemia represents a therapeutic challenge in patients with myelofibrosis (MF). Activin receptor-like kinase-2 (ALK2) activation is associated with elevated hepcidin, which may contribute to anemia of chronic disease and anemia due to hematologic malignancies, and is associated with increased transfusion rate and reduced overall survival.
Aims
This phase 1/2, open-label, multicenter, dose-escalation/dose-expansion study will evaluate the safety and tolerability of INCB000928, a potent, highly selective, ALK2 inhibitor, as monotherapy or combined with ruxolitinib in patients with anemia due to MF (INCB 00928-104; NCT04455841).
Methods
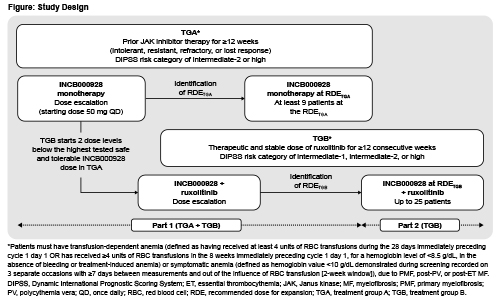
Patients with histologically confirmed primary or secondary MF presenting with symptomatic anemia or transfusion dependence will receive oral INCB000928 either alone (treatment group [TG] A) or combined with ruxolitinib (TGB; Figure). In TGA, patients must have intermediate (INT)-2 or high-risk (per Dynamic International Prognostic Scoring System [DIPSS]) disease that is intolerant, resistant, refractory, or has lost response to prior JAK inhibitor therapy (≥12 weeks). In TGB, patients must have received stable ruxolitinib therapy for ≥12 consecutive weeks before first dose and have INT-1/INT-2, or high-risk disease. Eligibility also requires age ≥18 years, platelet count ≥75 × 109/L, ECOG PS 0–1 (dose-escalation) or 0–2 (dose-expansion), life expectancy >6 months, no other hematologic malignancy, no prior stem cell transplantation, no major surgery within 28 days, and no prior disease-directed therapy within 5 half-lives or 28 days of first dose. In part 1 (dose escalation), TGA will receive INCB000928 monotherapy starting at 50 mg/day (28-day cycles) (Figure). Dose-escalation will follow a Bayesian optimal-interval design to determine the maximum tolerated dose (MTD) and/or recommended dose for expansion (RDE). Dose increases not exceeding 2-fold will continue until grade ≥2 treatment-related toxicity occurs. TGB will receive INCB000928 plus ruxolitinib; INCB000928 dose escalation will start 2 dose levels below the highest dose determined to be safe and tolerable in TGA. In part 2 (dose expansion), the RDE in TGB will be evaluated in combination with ruxolitinib in ~25 patients; treatment will continue for ≤12 months, and may continue if clinically beneficial and no progression occurs. Primary objective is to determine the safety and tolerability of INCB000928 as monotherapy or combined with ruxolitinib. Secondary objectives are to determine the efficacy of INCB000928 as monotherapy or combined with ruxolitinib (anemia response, duration of anemia response, mean change from baseline in hemoglobin, and RBC transfusion rate through weeks 24 and 48); evaluate INCB000928 pharmacokinetics; and evaluate effects of INCB000928 as monotherapy or combined with ruxolitinib on hepcidin levels and other measures of iron homeostasis and erythropoiesis. Sites are opening in the United States and Europe.
Results
Not applicable
Conclusion
Not applicable
Keyword(s): Anemia, Janus Kinase inhibitor, Kinase inhibitor, Myelofibrosis
Abstract: PB1713
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Anemia represents a therapeutic challenge in patients with myelofibrosis (MF). Activin receptor-like kinase-2 (ALK2) activation is associated with elevated hepcidin, which may contribute to anemia of chronic disease and anemia due to hematologic malignancies, and is associated with increased transfusion rate and reduced overall survival.
Aims
This phase 1/2, open-label, multicenter, dose-escalation/dose-expansion study will evaluate the safety and tolerability of INCB000928, a potent, highly selective, ALK2 inhibitor, as monotherapy or combined with ruxolitinib in patients with anemia due to MF (INCB 00928-104; NCT04455841).
Methods
Patients with histologically confirmed primary or secondary MF presenting with symptomatic anemia or transfusion dependence will receive oral INCB000928 either alone (treatment group [TG] A) or combined with ruxolitinib (TGB; Figure). In TGA, patients must have intermediate (INT)-2 or high-risk (per Dynamic International Prognostic Scoring System [DIPSS]) disease that is intolerant, resistant, refractory, or has lost response to prior JAK inhibitor therapy (≥12 weeks). In TGB, patients must have received stable ruxolitinib therapy for ≥12 consecutive weeks before first dose and have INT-1/INT-2, or high-risk disease. Eligibility also requires age ≥18 years, platelet count ≥75 × 109/L, ECOG PS 0–1 (dose-escalation) or 0–2 (dose-expansion), life expectancy >6 months, no other hematologic malignancy, no prior stem cell transplantation, no major surgery within 28 days, and no prior disease-directed therapy within 5 half-lives or 28 days of first dose. In part 1 (dose escalation), TGA will receive INCB000928 monotherapy starting at 50 mg/day (28-day cycles) (Figure). Dose-escalation will follow a Bayesian optimal-interval design to determine the maximum tolerated dose (MTD) and/or recommended dose for expansion (RDE). Dose increases not exceeding 2-fold will continue until grade ≥2 treatment-related toxicity occurs. TGB will receive INCB000928 plus ruxolitinib; INCB000928 dose escalation will start 2 dose levels below the highest dose determined to be safe and tolerable in TGA. In part 2 (dose expansion), the RDE in TGB will be evaluated in combination with ruxolitinib in ~25 patients; treatment will continue for ≤12 months, and may continue if clinically beneficial and no progression occurs. Primary objective is to determine the safety and tolerability of INCB000928 as monotherapy or combined with ruxolitinib. Secondary objectives are to determine the efficacy of INCB000928 as monotherapy or combined with ruxolitinib (anemia response, duration of anemia response, mean change from baseline in hemoglobin, and RBC transfusion rate through weeks 24 and 48); evaluate INCB000928 pharmacokinetics; and evaluate effects of INCB000928 as monotherapy or combined with ruxolitinib on hepcidin levels and other measures of iron homeostasis and erythropoiesis. Sites are opening in the United States and Europe.
Results
Not applicable

Conclusion
Not applicable
Keyword(s): Anemia, Janus Kinase inhibitor, Kinase inhibitor, Myelofibrosis


