
Contributions
Abstract: PB1481
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
The standard of care treatment (tx) in PNH is based on complement C5 inhibition with anti-C5 monoclonal antibodies (MAbs). Anti-C5 MAbs are generally effective at treating intravascular hemolysis (IVH) but unmet medical needs remain. Hematological responses to anti-C5 antibody tx can be heterogenous and a substantial number of pts experience residual anemia associated with breakthrough hemolysis (BTH). Anti-C5 MAbs are also not available in all countries and, where available, pt access can be limited. Iptacopan (LNP023) is a first-in-class, oral, small-molecule, selective, and reversible complement factor B inhibitor that inhibits both IVH and extravascular hemolysis (EVH). In an ongoing Ph2 study (NCT03439839), iptacopan has demonstrated improvements in both hematological response and biomarkers of disease activity in adult pts with PNH who had active hemolysis despite tx with eculizumab. These findings warrant further Ph3 studies to assess the efficacy of iptacopan in a wider population of pts with PNH, including those who are naïve to anti-C5 MAbs tx.
Aims
The Ph3, single-arm, open-label APPOINT-PNH study (CLNP023C12301) will assess the efficacy and safety of iptacopan in adult pts with PNH who are naïve to complement inhibitor therapy, including anti-C5 MAbs.
Methods
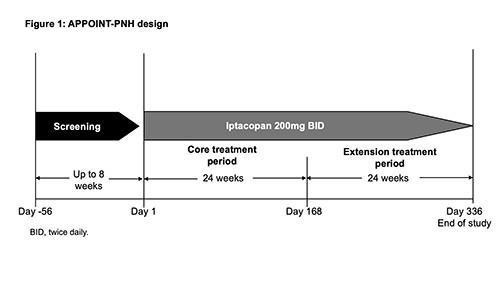
The study comprises an ≤8-wk screening period; a 24-wk, single-arm, open-label core tx period; and a 24-wk, open-label tx extension period (Fig. 1). On completion of the Wk 24 visit, pts who show benefit from iptacopan tx can enter the extension period. Eligible adult pts (N≈40; ≥18 years old) must have a confirmed diagnosis of PNH (red blood cells [RBCs] and white blood cells [granulocyte/monocyte] clone size ≥10%); anemia (mean Hb <10g/dL) and active hemolysis (lactate dehydrogenase [LDH] >1.5×upper limit of normal). Key exclusion criteria: prior tx with a complement inhibitor; known or suspected hereditary complement deficiency; history of hematopoietic stem cell transplantation; laboratory evidence of bone marrow failure; active, systemic infection ≤14 days preceding study drug tx; and history of recurrent, invasive infections caused by encapsulated microorganisms. The primary objective is to assess hematologic response, defined as achieving a sustained increase in Hb levels ≥2g/dL (in the absence of RBC transfusions). The primary endpoint is to evaluate the proportion of pts achieving an increase from baseline (BL) in Hb levels ≥2g/dL, between D126–D168 (in the absence of RBC transfusions between D14–D168). The analysis of the primary endpoint will be a logistic regression model including BL covariates: sex, age (categorical), Hb >8g/dL, and presence of transfusion dependence at enrollment. Secondary endpoints include: proportion of pts reaching Hb levels ≥12g/dL; absence of RBC transfusions; changes from BL in Hb levels; changes in patient-reported fatigue scores (FACIT-Fatigue); changes from BL in reticulocytes; % change from BL in LDH; occurrence of BTH; occurrence of major adverse vascular events (including thrombosis). Additional analyses include safety assessments; evolution of PNH clone size and C3d deposition on RBCs; and other PROs (PGI-S, EORTC QLQ-C30, EQ‑5D-5L).
Results
NA.
Conclusion
Ph2 study data suggest that iptacopan may control EVH and IVH, thereby reducing anemia in pts with PNH previously treated with anti-C5 MAbs. Data from a further Ph2 study of iptacopan in patients with treatment-naïve PNH (NCT03896152) are awaited. Ph3 studies to assess the efficacy of iptacopan in a wider population of pts with PNH are planned.
Keyword(s): Anemia, Hemolysis, PNH
Abstract: PB1481
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
The standard of care treatment (tx) in PNH is based on complement C5 inhibition with anti-C5 monoclonal antibodies (MAbs). Anti-C5 MAbs are generally effective at treating intravascular hemolysis (IVH) but unmet medical needs remain. Hematological responses to anti-C5 antibody tx can be heterogenous and a substantial number of pts experience residual anemia associated with breakthrough hemolysis (BTH). Anti-C5 MAbs are also not available in all countries and, where available, pt access can be limited. Iptacopan (LNP023) is a first-in-class, oral, small-molecule, selective, and reversible complement factor B inhibitor that inhibits both IVH and extravascular hemolysis (EVH). In an ongoing Ph2 study (NCT03439839), iptacopan has demonstrated improvements in both hematological response and biomarkers of disease activity in adult pts with PNH who had active hemolysis despite tx with eculizumab. These findings warrant further Ph3 studies to assess the efficacy of iptacopan in a wider population of pts with PNH, including those who are naïve to anti-C5 MAbs tx.
Aims
The Ph3, single-arm, open-label APPOINT-PNH study (CLNP023C12301) will assess the efficacy and safety of iptacopan in adult pts with PNH who are naïve to complement inhibitor therapy, including anti-C5 MAbs.
Methods
The study comprises an ≤8-wk screening period; a 24-wk, single-arm, open-label core tx period; and a 24-wk, open-label tx extension period (Fig. 1). On completion of the Wk 24 visit, pts who show benefit from iptacopan tx can enter the extension period. Eligible adult pts (N≈40; ≥18 years old) must have a confirmed diagnosis of PNH (red blood cells [RBCs] and white blood cells [granulocyte/monocyte] clone size ≥10%); anemia (mean Hb <10g/dL) and active hemolysis (lactate dehydrogenase [LDH] >1.5×upper limit of normal). Key exclusion criteria: prior tx with a complement inhibitor; known or suspected hereditary complement deficiency; history of hematopoietic stem cell transplantation; laboratory evidence of bone marrow failure; active, systemic infection ≤14 days preceding study drug tx; and history of recurrent, invasive infections caused by encapsulated microorganisms. The primary objective is to assess hematologic response, defined as achieving a sustained increase in Hb levels ≥2g/dL (in the absence of RBC transfusions). The primary endpoint is to evaluate the proportion of pts achieving an increase from baseline (BL) in Hb levels ≥2g/dL, between D126–D168 (in the absence of RBC transfusions between D14–D168). The analysis of the primary endpoint will be a logistic regression model including BL covariates: sex, age (categorical), Hb >8g/dL, and presence of transfusion dependence at enrollment. Secondary endpoints include: proportion of pts reaching Hb levels ≥12g/dL; absence of RBC transfusions; changes from BL in Hb levels; changes in patient-reported fatigue scores (FACIT-Fatigue); changes from BL in reticulocytes; % change from BL in LDH; occurrence of BTH; occurrence of major adverse vascular events (including thrombosis). Additional analyses include safety assessments; evolution of PNH clone size and C3d deposition on RBCs; and other PROs (PGI-S, EORTC QLQ-C30, EQ‑5D-5L).
Results
NA.

Conclusion
Ph2 study data suggest that iptacopan may control EVH and IVH, thereby reducing anemia in pts with PNH previously treated with anti-C5 MAbs. Data from a further Ph2 study of iptacopan in patients with treatment-naïve PNH (NCT03896152) are awaited. Ph3 studies to assess the efficacy of iptacopan in a wider population of pts with PNH are planned.
Keyword(s): Anemia, Hemolysis, PNH


